SPG302 Reverses Synaptic and Cognitive Deficits Without Altering Amyloid or Tau Pathology in a Transgenic Model of Alzheimer's Disease
- PMID: 34738197
- PMCID: PMC8804111
- DOI: 10.1007/s13311-021-01143-1
SPG302 Reverses Synaptic and Cognitive Deficits Without Altering Amyloid or Tau Pathology in a Transgenic Model of Alzheimer's Disease
Abstract
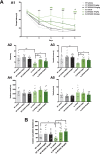
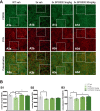
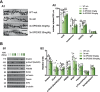
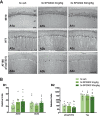
Alzheimer's disease (AD) is conceptualized as a synaptic failure disorder in which loss of glutamatergic synapses is a major driver of cognitive decline. Thus, novel therapeutic strategies aimed at regenerating synapses may represent a promising approach to mitigate cognitive deficits in AD patients. At present, no disease-modifying drugs exist for AD, and approved therapies are palliative at best, lacking in the ability to reverse the synaptic failure. Here, we tested the efficacy of a novel synaptogenic small molecule, SPG302 - a 3rd-generation benzothiazole derivative that increases the density of axospinous glutamatergic synapses - in 3xTg-AD mice. Daily dosing of 3xTg-AD mice with SPG302 at 3 and 30 mg/kg (i.p.) for 4 weeks restored hippocampal synaptic density and improved cognitive function in hippocampal-dependent tasks. Mushroom and stubby spine profiles were increased by SPG302, and associated with enhanced expression of key postsynaptic proteins - including postsynaptic density protein 95 (PSD95), drebrin, and amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) - and increased colocalization of PSD95 with synaptophysin. Notably, SPG302 proved efficacious in this model without modifying Aβ and tau pathology. Thus, our study provides preclinical support for the idea that compounds capable of restoring synaptic density offer a viable strategy to reverse cognitive decline in AD.
Keywords: 3xTg-AD mice; Alzheimer’s disease; Dendritic spines; SPG302; Synaptic deficits; Synaptic markers.
© 2021. The Author(s).
Conflict of interest statement
P.W.V., S.T.S. and V.F.S declare that they are members of the executive team of Spinogenix where they are developing SPG compounds as therapeutics for synaptopathies. All other authors declare no competing interests.
Figures
References
-
- 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement [Internet]. John Wiley and Sons Inc.; 2020 [cited 2020 Dec 9];16:391–460. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/alz.12068 - DOI
-
- Elmaleh DR, Farlow MR, Conti PS, Tompkins RG, Kundakovic L, Tanzi RE. Developing Effective Alzheimer’s Disease Therapies: Clinical Experience and Future Directions [Internet]. J. Alzheimer’s Dis. IOS Press; 2019 [cited 2020 Dec 9]. p. 715–32. Available from: https://pubmed.ncbi.nlm.nih.gov/31476157/ - PMC - PubMed
-
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science (80-. ). 2002. p. 789–91. - PubMed
-
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: Correlation with cognitive severity. Ann Neurol [Internet]. Ann Neurol; 1990 [cited 2020 Dec 9];27:457–64. Available from: https://pubmed.ncbi.nlm.nih.gov/2360787/ - PubMed

