Tunneling nanotube formation promotes survival against 5-fluorouracil in MCF-7 breast cancer cells
- PMID: 34738322
- PMCID: PMC8727926
- DOI: 10.1002/2211-5463.13324
Tunneling nanotube formation promotes survival against 5-fluorouracil in MCF-7 breast cancer cells
Abstract
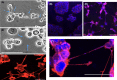
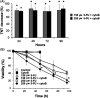
Tunneling nanotubes (TNTs) are F-actin-based open-ended tubular extensions that form following stresses, such as nutritional deprivation and oxidative stress. The chemotherapy agent 5-fluorouracil (5-FU) represents a significant stressor to cancer cells and induces thymidine deficiency, a state similar to nutritional deprivation. However, the ability of 5-FU to induce TNT formation in cancer cells and potentially enhance survival has not been explored. In this study, we examined whether 5-FU can induce TNT formation in MCF-7 breast cancer cells. Cytotoxic doses of 5-FU (150-350 μm) were observed to significantly induce TNT formation beginning at 24 h after exposure. TNTs formed following 5-FU treatment probably originated as extensions of gap junctions as MCF-7 cells detach from cell clusters. TNTs act as conduits for exchange of cellular components and we observed mitochondrial exchange through TNTs following 5-FU treatment. 5-FU-induced TNT formation was inhibited by over 80% following treatment with the F-actin-depolymerizing agent, cytochalasin B (cytoB). The inhibition of TNTs by cytoB corresponded with increased 5-FU-induced cytotoxicity by 30-62% starting at 48 h, suggesting TNT formation aides in MCF-7 cell survival against 5-FU. Two other widely used chemotherapy agents, docetaxel and doxorubicin induced TNT formation at much lower levels than 5-FU. Our work suggests that the therapeutic targeting of TNTs may increase 5-FU chemotherapy efficacy and decrease drug resistance in cancer cells, and these findings merits further investigation.
Keywords: 5-fluorouracil; chemotherapy; cytochalasin B; doxorubicin; drug resistance; tunneling nanotubes.
© 2021 The Authors. FEBS Open Bio published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–10. - PubMed
-
- Abounit S, Zurzolo C. Wiring through tunneling nanotubes–from electrical signals to organelle transfer. J Cell Sci. 2012;125:1089–98. - PubMed
-
- Mittal R, Karhu E, Wang JS, Delgado S, Zukerman R, Mittal J, et al. Cell communication by tunneling nanotubes: implications in disease and therapeutic applications. J Cell Physiol. 2019;234:1130–46. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

