Microbiota-derived extracellular vesicles in interkingdom communication in the gut
- PMID: 34738337
- PMCID: PMC8568775
- DOI: 10.1002/jev2.12161
Microbiota-derived extracellular vesicles in interkingdom communication in the gut
Abstract
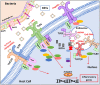
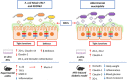
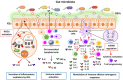
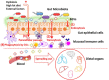
The intestine is fundamental in controlling human health. Intestinal epithelial and immune cells are continuously exposed to millions of microbes that greatly impact on intestinal epithelial barrier and immune function. This microbial community, known as gut microbiota, is now recognized as an important partner of the human being that actively contribute to essential functions of the intestine but also of distal organs. In the gut ecosystem, bidirectional microbiota-host communication does not involve direct cell contacts. Both microbiota and host-derived extracellular vesicles (EVs) are key players of such interkingdom crosstalk. There is now accumulating body of evidence that bacterial secreted vesicles mediate microbiota functions by transporting and delivering into host cells effector molecules that modulate host signalling pathways and cell processes. Consequently, vesicles released by the gut microbiota may have great influence on health and disease. Here we review current knowledge on microbiota EVs and specifically highlight their role in controlling host metabolism, intestinal barrier integrity and immune training.
Keywords: bacterial membrane vesicles; gut microbiota; gut permeability; immune regulation; intestinal homeostasis; probiotics.
© 2021 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
AUTHOR CONTRIBUTIONS
Conceptualization, J.B and L.B; investigation, N.D.G, writing—original draft preparation, N.D.G.; writing—review and editing, L.B and J.B; supervision L.B; project administration, J.B; funding acquisition, L.B and J.B.
Figures

References
-
- Ahmadi Badi, S. , Moshiri, A. , Ettehad Marvasti, F., Mojtahedzadeh, M. , Kazemi, V. , & Siadat, S. D. (2020). Extraction and evaluation of outer membrane vesicles from two important gut microbiota members, Bacteroides fragilis and Bacteroides thetaiotaomicron . Cell Journal, 22(3), 344–349. 10.22074/cellj.2020.6499 - DOI - PMC - PubMed
-
- Al‐Nedawi, K. , Mian, M. F. , Hossain, N. , Karimi, K. , Mao, Y.‐K. , Forsythe, P. , Min, K. K. , Stanisz, A. M. , Kunze, W. A. , & Bienenstock, J. (2015). Gut commensal microvesicles reproduce parent bacterial signals to host immune and enteric nervous systems. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology, 29(2), 684–695. 10.1096/fj.14-259721 - DOI - PubMed
-
- Alvarez, C.‐S. , Giménez, R. , Cañas, M.‐A. , Vera, R. , Díaz‐Garrido, N. , Badia, J. , & Baldomà, L. (2019). Extracellular vesicles and soluble factors secreted by Escherichia coli Nissle 1917 and ECOR63 protect against enteropathogenic E. coli‐induced intestinal epithelial barrier dysfunction. BMC Microbiology, 19(1). 10.1186/s12866-019-1534-3 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

