Long noncoding RNA NR2F1-AS1 plays a carcinogenic role in gastric cancer by recruiting transcriptional factor SPI1 to upregulate ST8SIA1 expression
- PMID: 34738863
- PMCID: PMC8810033
- DOI: 10.1080/21655979.2021.2001168
Long noncoding RNA NR2F1-AS1 plays a carcinogenic role in gastric cancer by recruiting transcriptional factor SPI1 to upregulate ST8SIA1 expression
Abstract
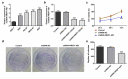
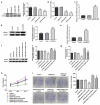
Gastric cancer (GC) is a highly malignant solid tumor of the digestive tract, which is associated with a high mortality rate. Long non-coding RNA (lncRNA) nuclear receptor subfamily 2 group F member 1 antisense RNA 1 (NR2F1-AS1) has been reported to exert a tumor-promoting effect in some types of cancer. The present study aimed to investigate the role of NR2F1-AS1 in GC. The expression levels of NR2F1-AS1 and its potential target gene were measured in GC cell lines. Bioinformatics analysis, an RNA immunoprecipitation assay and a chromatin immunoprecipitation assay were used to determine the binding relationship between NR2F1-AS1 and downstream genes. The effect of NR2F1-AS1 regulatory axis on AGC cell viability, proliferation, migration, invasion and epithelial-mesenchymal transition was evaluated. The results of the present study revealed that the knockdown of NR2F1-AS1 inhibited the proliferation, invasion and migration of GC cells. NR2F1-AS1 also upregulated the expression levels of ST8SIA1 by recruiting transcriptional factor SPI1. Thus, the effects of the knockdown of NR2F1-AS1 on GC cell functions were suggested to occur via regulation of ST8SIA1. In conclusion, the findings of the current study indicated that NR2F1-AS1 may promote the proliferation, invasion and migration of GC cells by recruiting SPI1, to upregulate ST8SIA1 expression. Thus, the regulation of their expression levels may provide a novel direction for the treatment of GC.
Keywords: Gastric cancer; LncRNA NR2F1-AS; SPI1; ST8SIA1.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




Similar articles
-
The EMT-induced lncRNA NR2F1-AS1 positively modulates NR2F1 expression and drives gastric cancer via miR-29a-3p/VAMP7 axis.Cell Death Dis. 2022 Jan 26;13(1):84. doi: 10.1038/s41419-022-04540-2. Cell Death Dis. 2022. PMID: 35082283 Free PMC article.
-
Hypoxia-induced long noncoding RNA NR2F1-AS1 maintains pancreatic cancer proliferation, migration, and invasion by activating the NR2F1/AKT/mTOR axis.Cell Death Dis. 2022 Mar 14;13(3):232. doi: 10.1038/s41419-022-04669-0. Cell Death Dis. 2022. PMID: 35283481 Free PMC article.
-
STAT3 activates the transcription of lncRNA NR2F1-AS1 to promote the progression of melanoma via regulating the miR-493-5p/GOLM1 axis.J Gene Med. 2021 Jul;23(7):e3338. doi: 10.1002/jgm.3338. Epub 2021 Apr 27. J Gene Med. 2021. PMID: 33822440
-
NR2F1-AS1: A Functional Long Noncoding RNA in Tumorigenesis.Curr Med Chem. 2023;30(37):4266-4276. doi: 10.2174/0929867330666230112165503. Curr Med Chem. 2023. PMID: 36644870 Review.
-
The emerging role of NR2F1-AS1 in the tumorigenesis and progression of human cancer.Pathol Res Pract. 2022 Jul;235:153938. doi: 10.1016/j.prp.2022.153938. Epub 2022 May 6. Pathol Res Pract. 2022. PMID: 35552086 Review.
Cited by
-
LINC01004-SPI1 axis-activated SIGLEC9 in tumor-associated macrophages induces radioresistance and the formation of immunosuppressive tumor microenvironment in esophageal squamous cell carcinoma.Cancer Immunol Immunother. 2023 Jun;72(6):1835-1851. doi: 10.1007/s00262-022-03364-5. Epub 2023 Jan 23. Cancer Immunol Immunother. 2023. PMID: 36688997 Free PMC article.
-
SPI1 activates TGF-β1/PI3K/Akt signaling through transcriptional upregulation of FKBP12 to support the mesenchymal phenotype of glioma stem cells.Brain Pathol. 2024 May;34(3):e13217. doi: 10.1111/bpa.13217. Epub 2023 Oct 22. Brain Pathol. 2024. PMID: 37865975 Free PMC article.
-
The biological role and immunotherapy of gangliosides and GD3 synthase in cancers.Front Cell Dev Biol. 2023 Feb 7;11:1076862. doi: 10.3389/fcell.2023.1076862. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36824365 Free PMC article. Review.
-
The short peptide encoded by long non-coding RNA RNF217-AS1 inhibits stomach cancer tumorigenesis, macrophage recruitment, and pro-inflammatory responses.Amino Acids. 2024 Jul 15;56(1):45. doi: 10.1007/s00726-024-03404-7. Amino Acids. 2024. PMID: 39007996 Free PMC article.
-
EIF4A3 induced circABCA5 promotes the gastric cancer progression by SPI1 mediated IL6/JAK2/STAT3 signaling.Am J Cancer Res. 2023 Feb 15;13(2):602-622. eCollection 2023. Am J Cancer Res. 2023. PMID: 36895988 Free PMC article.
References
-
- Paredes-Torres OR, Garcia-Ruiz L, Luna-Abanto J, et al. Risk factors associated with postoperative morbidity and mortality in D2 radical gastrectomy for gastric cancer. Rev Gastroenterol Mex. 2021. Mar 19;S0375-0906(21):00013–6. - PubMed
-
- Morais S, Antunes L, Bento MJ, et al. Second primary gastric cancers in a region with an overall high risk of gastric cancer. Gac Sanit. 2020. Jul–Aug;34(4):393–398. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
