Screening in High Schools to Identify, Evaluate, and Lower Depression Among Adolescents: A Randomized Clinical Trial
- PMID: 34739064
- PMCID: PMC8571659
- DOI: 10.1001/jamanetworkopen.2021.31836
Screening in High Schools to Identify, Evaluate, and Lower Depression Among Adolescents: A Randomized Clinical Trial
Abstract
Importance: Adolescent major depressive disorder (MDD) prevalence has nearly doubled in the past decade. The US Preventive Services Task Force endorses universal adolescent MDD screening in primary care; however, most adolescents lack preventive health care, resulting in worsening disparities in MDD screening and treatment.
Objective: To evaluate the effectiveness of universal adolescent MDD screening in the school setting in an effort to reduce disparities and improve MDD identification and treatment initiation.
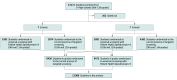
Design, setting, and participants: This randomized clinical trial, conducted from November 6, 2018, to November 20, 2020, compared the usual school practice of targeted or selected screening based on observable behaviors of concern with universal MDD screening. Students within an identified school were randomized by grade to 1 of the 2 study groups. Study groups were compared using mixed-effects logistic regression. Participants included students in grades 9 through 12 enrolled at 1 of the 14 participating Pennsylvania public high schools.
Interventions: In targeted screening, students with behaviors prompting concern for MDD were referred to the Student Assistance Program (SAP), mandated in all Pennsylvania schools. The SAP determined follow-up recommendations. In universal screening, all students completed the Patient Health Questionnaire-9 (PHQ-9); students with positive scores proceeded to SAP. The universal screening group could also have targeted referral to SAP for concerning behavior independent of the PHQ-9.
Main outcomes and measures: The primary outcome was initiation of MDD treatment or services based on data collected by school SAP teams during the academic year.
Results: A total of 12 909 students were included (median age, 16 years [range, 13-21 years]; 6963 male [53.9%]), of whom 2687 (20.8%) were Hispanic, 2891 (22.4%) were non-Hispanic Black, 5842 (45.3%) were non-Hispanic White, and 1489 (11.5%) were multiracial or of other race or ethnicity. A total of 6473 students (50.1%) were randomized to universal screening, and 6436 (49.9%) were randomized to targeted screening. Adolescents in the universal screening group had 5.92 times higher odds (95% CI, 5.07-6.93) of being identified with MDD symptoms, 3.30 times higher odds (95% CI, 2.49-4.38) of SAP confirming follow-up needs, and 2.07 times higher odds (95% CI, 1.39-3.10) of initiating MDD treatment. No differences were identified in initiation for planned subgroup analyses by sex or race and ethnicity.
Conclusions and relevance: In this randomized clinical trial, universal school-based MDD screening successfully increased identification of MDD symptoms and treatment initiation among adolescents, confirming the value of this approach to address this rising public health concern.
Trial registration: ClinicalTrials.gov identifier: NCT03716869.
Conflict of interest statement
Comment in
-
Universal Depression Screening in Schools-Promises and Challenges in Addressing Adolescent Mental Health Need.JAMA Netw Open. 2021 Nov 1;4(11):e2132858. doi: 10.1001/jamanetworkopen.2021.32858. JAMA Netw Open. 2021. PMID: 34739068 No abstract available.
References
-
- Healthy People.gov. Mental health and mental disorders. Accessed February 24, 2021. https://www.healthypeople.gov/2020/data-search/Search-the-Data?nid=4813
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous