Venetoclax plus azacitidine in Japanese patients with untreated acute myeloid leukemia ineligible for intensive chemotherapy
- PMID: 34739075
- PMCID: PMC9242001
- DOI: 10.1093/jjco/hyab170
Venetoclax plus azacitidine in Japanese patients with untreated acute myeloid leukemia ineligible for intensive chemotherapy
Erratum in
-
Correction to: Venetoclax plus azacitidine in Japanese patients with untreated acute myeloid leukemia ineligible for intensive chemotherapy.Jpn J Clin Oncol. 2023 Apr 29;53(5):456. doi: 10.1093/jjco/hyad030. Jpn J Clin Oncol. 2023. PMID: 36971289 Free PMC article. No abstract available.
Abstract
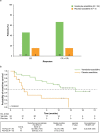
Background: The phase 3 VIALE-A trial (NCT02993523) reported that venetoclax-azacitidine significantly prolonged overall survival compared with placebo-azacitidine in patients with newly diagnosed acute myeloid leukemia ineligible for intensive chemotherapy. Herein, efficacy and safety of venetoclax-azacitidine are analyzed in the Japanese subgroup of VIALE-A patients.
Methods: Eligible Japanese patients were randomized 2:1 to venetoclax-azacitidine (N = 24) or placebo-azacitidine (N = 13). Primary endpoints for Japan were overall survival and complete response (CR) + CR with incomplete hematologic recovery (CRi). Venetoclax (target dose 400 mg) was given orally once daily. Azacitidine (75 mg/m2) was administered subcutaneously or intravenously on Days 1-7 of each 28-day cycle.
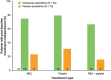
Results: Median follow-up was 16.3 months (range, 1.0-20.3). Median overall survival was not reached with venetoclax-azacitidine (hazard ratio 0.409 and 95% confidence interval: 0.151, 1.109); overall survival estimate was higher with venetoclax-azacitidine than placebo-azacitidine at 12 (67 and 46%) and 18 months (57 and 31%), respectively. CR and CRi rates were 67% with venetoclax-azacitidine and 15% with placebo-azacitidine. Most common any-grade adverse events were febrile neutropenia (79 and 39%), thrombocytopenia (54 and 77%), constipation (54 and 54%) and decreased appetite (54 and 38%) in the venetoclax-azacitidine and placebo-azacitidine arms, respectively. Only 1 patient in the venetoclax-azacitidine arm, and no patients in the placebo-azacitidine arm, had grade 4 febrile neutropenia that led to treatment discontinuation.
Conclusions: This Japanese subgroup analysis of VIALE-A demonstrates comparable safety and efficacy outcomes compared with the global study and supports venetoclax-azacitidine as first-line standard-of-care for Japanese treatment-naive patients with acute myeloid leukemia who are ineligible for intensive chemotherapy.
Keywords: Japan; VIALE-A; acute myeloid leukemia; azacitidine; venetoclax.
© The Author(s) 2021. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Deschler B, Lübbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer 2006;107:2099–107. - PubMed
-
- Miyawaki S. Clinical studies of acute myeloid leukemia in the Japan Adult Leukemia Study Group. Int J Hematol 2012;96:171–7. - PubMed
-
- Cancer Information Service . Cancer Statistics in Japan; Table Download. 2. Incidence (National Estimates). https://ganjoho.jp/en/professional/statistics/table_download.html (4 August 2021, date last accessed).
-
- Cancer Information Service . Cancer Statistics in Japan; Table Download. 5. Survival. https://ganjoho.jp/en/professional/statistics/table_download.html (4 August 2021, date last accessed).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

