TSC1 loss increases risk for tauopathy by inducing tau acetylation and preventing tau clearance via chaperone-mediated autophagy
- PMID: 34739309
- PMCID: PMC8570595
- DOI: 10.1126/sciadv.abg3897
TSC1 loss increases risk for tauopathy by inducing tau acetylation and preventing tau clearance via chaperone-mediated autophagy
Abstract
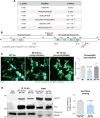
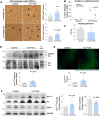
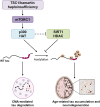
Age-associated neurodegenerative disorders demonstrating tau-laden intracellular inclusions are known as tauopathies. We previously linked a loss-of-function mutation in the TSC1 gene to tau accumulation and frontotemporal lobar degeneration. Now, we have identified genetic variants in TSC1 that decrease TSC1/hamartin levels and predispose to tauopathies such as Alzheimer’s disease and progressive supranuclear palsy. Cellular and murine models of TSC1 haploinsufficiency, as well as human brains carrying a TSC1 risk variant, accumulated tau protein that exhibited aberrant acetylation. This acetylation hindered tau degradation via chaperone-mediated autophagy, thereby leading to its accumulation. Aberrant tau acetylation in TSC1 haploinsufficiency resulted from the dysregulation of both p300 acetyltransferase and SIRT1 deacetylase. Pharmacological modulation of either enzyme restored tau levels. This study substantiates TSC1 as a novel tauopathy risk gene and includes TSC1 haploinsufficiency as a genetic model for tauopathies. In addition, these findings promote tau acetylation as a rational target for tauopathy therapeutics and diagnostic.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

