The metabolic adaptation evoked by arginine enhances the effect of radiation in brain metastases
- PMID: 34739311
- PMCID: PMC8570607
- DOI: 10.1126/sciadv.abg1964
The metabolic adaptation evoked by arginine enhances the effect of radiation in brain metastases
Abstract
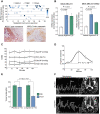
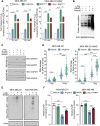
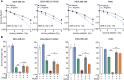
Selected patients with brain metastases (BM) are candidates for radiotherapy. A lactatogenic metabolism, common in BM, has been associated with radioresistance. We demonstrated that BM express nitric oxide (NO) synthase 2 and that administration of its substrate l-arginine decreases tumor lactate in BM patients. In a placebo-controlled trial, we showed that administration of l-arginine before each fraction enhanced the effect of radiation, improving the control of BM. Studies in preclinical models demonstrated that l-arginine radiosensitization is a NO-mediated mechanism secondary to the metabolic adaptation induced in cancer cells. We showed that the decrease in tumor lactate was a consequence of reduced glycolysis that also impacted ATP and NAD+ levels. These effects were associated with NO-dependent inhibition of GAPDH and hyperactivation of PARP upon nitrosative DNA damage. These metabolic changes ultimately impaired the repair of DNA damage induced by radiation in cancer cells while greatly sparing tumor-infiltrating lymphocytes.
Figures




References
-
- Kamar F. G., Posner J. B., Brain metastases. Semin. Neurol. 30, 217–235 (2010). - PubMed
-
- Illum H., Wang D. H., Dowell J. E., Hittson W. J., Torrisi J. R., Meyer J., Huerta S., Phase I dose escalation trial of nitroglycerin in addition to 5-fluorouracil and radiation therapy for neoadjuvant treatment of operable rectal cancer. Surgery 158, 460–465 (2015). - PubMed
-
- Gao X., Saha D., Kapur P., Anthony T., Livingston E. H., Huerta S., Radiosensitization of HT-29 cells and xenografts by the nitric oxide donor DETANONOate. J. Surg. Oncol. 100, 149–158 (2009). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

