Development of the Swiss Database for dosing medicinal products in pediatrics
- PMID: 34739591
- PMCID: PMC8897330
- DOI: 10.1007/s00431-021-04304-8
Development of the Swiss Database for dosing medicinal products in pediatrics
Erratum in
-
Correction to: Development of the Swiss database for dosing medicinal products in pediatrics.Eur J Pediatr. 2022 Mar;181(3):1233. doi: 10.1007/s00431-021-04328-0. Eur J Pediatr. 2022. PMID: 34870750 Free PMC article. No abstract available.
Abstract
In daily paediatrics, drugs are commonly used off-label, as they are not approved for children. Approval is lacking because the required clinical studies were limited to adults in the past. Without clinical studies, evidence-based recommendations for drug use in children are limited. Information on off-label drug dosing in children can be found in different handbooks, databases and scientific publications but the dosing recommendations can differ considerably. To improve safety and efficacy of drugs prescribed to children and to assist the prescribers, stakeholders in Swiss paediatrics started a pilot project, supported by the Federal Office of Public Health, with the aim to create a database, providing healthcare professionals with so called "harmonised" dosage recommendations based on the latest available scientific evidence and best clinical practice. A standardised process for dosage harmonisation between paediatric experts was defined, guided and documented in an electronic tool, developed for this purpose. As proof of principle, a total of 102 dosage recommendations for 30 different drugs have been nationally harmonised in the pilot phase considering the current scientific literature and the approval of the most experienced national experts in the field.Conclusion: This approach paved the way for unified national dosage recommendations for children. Reaching the project's milestones fulfilled the prerequisites for funding and starting regular operation of SwissPedDose in 2018. Since then, the database was extended with recommendations for 100 additional drugs. What is Known: • Prescribing off-label is a common practice among paediatricians, as many drugs are still not authorised for use in children. • Some countries developed national drug formularies providing off-label dosage recommendations. What is New: • Comparison of published dosage recommendations in known drug handbooks and online databases show substantial differences and heterogeneity, revealing the need for harmonisation. • The design of a tool for standardised harmonisation of dosage recommendations, based on information collected on currently applied dosages, latest scientific evidence and the approval of experts.
Keywords: Children; Drug safety; Off-label use; Prescribing; Web-based software.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests. Only ST who is employed by the FOPH has a financial relationship with the organizations that sponsored the pilot project to declare.
Figures
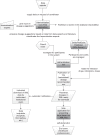

References
-
- Government U.S. Best Pharmaceuticals for Children Act (2002) available at https://www.congress.gov/107/plaws/publ109/PLAW-107publ109.pdf (last accessed 06 November 2019)
-
- Government U.S. Pediatric Research Equity Act of 2003 (2003) available at https://www.congress.gov/108/plaws/publ155/PLAW-108publ155.pdf (last accessed 06 November 2019)
-
- Union E.P.a.C.o.t.E., Regulation (EC) No 1901/2006 on medicinal products for paediatric use (2006) available at https://ec.europa.eu/health//sites/health/files/files/eudralex/vol-1/reg... (last accessed 30 October 2019)
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

