Barriers and facilitators to the uptake of new medicines into clinical practice: a systematic review
- PMID: 34740338
- PMCID: PMC8570007
- DOI: 10.1186/s12913-021-07196-4
Barriers and facilitators to the uptake of new medicines into clinical practice: a systematic review
Abstract
Background: Implementation and uptake of novel and cost-effective medicines can improve patient health outcomes and healthcare efficiency. However, the uptake of new medicines into practice faces a wide range of obstacles. Earlier reviews provided insights into determinants for new medicine uptake (such as medicine, prescriber, patient, organization, and external environment factors). However, the methodological approaches used had limitations (e.g., single author, narrative review, narrow search, no quality assessment of reviewed evidence). This systematic review aims to identify barriers and facilitators affecting the uptake of new medicines into clinical practice and identify areas for future research.
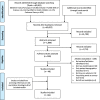
Method: A systematic search of literature was undertaken within seven databases: Medline, EMBASE, Web of Science, CINAHL, Cochrane Library, SCOPUS, and PsychINFO. Included in the review were qualitative, quantitative, and mixed-methods studies focused on adult participants (18 years and older) requiring or taking new medicine(s) for any condition, in the context of healthcare organizations and which identified factors affecting the uptake of new medicines. The methodological quality was assessed using QATSDD tool. A narrative synthesis of reported factors was conducted using framework analysis and a conceptual framework was utilised to group them.
Results: A total of 66 studies were included. Most studies (n = 62) were quantitative and used secondary data (n = 46) from various databases, e.g., insurance databases. The identified factors had a varied impact on the uptake of the different studied new medicines. Differently from earlier reviews, patient factors (patient education, engagement with treatment, therapy preferences), cost of new medicine, reimbursement and formulary conditions, and guidelines were suggested to influence the uptake. Also, the review highlighted that health economics, wider organizational factors, and underlying behaviours of adopters were not or under explored.
Conclusion: This systematic review has identified a broad range of factors affecting the uptake of new medicines within healthcare organizations, which were grouped into patient, prescriber, medicine, organizational, and external environment factors. This systematic review also identifies additional factors affecting new medicine use not reported in earlier reviews, which included patient influence and education level, cost of new medicines, formulary and reimbursement restrictions, and guidelines.
Registration: PROSPERO database (CRD42018108536).
Keywords: Healthcare organizations; Implementation; Innovation implementation; New medicines; Systematic review; Uptake.
© 2021. The Author(s).
Conflict of interest statement
The authors declare they have no competing interests.
Figures
References
-
- Ewbank L, Omojomolo D, Sullivan K, McKenna H. The rising cost of medicines to the NHS: what is the story? The King’s fund. 2018.
-
- World Health Organization. WHO Health Innovation Group. https://www.who.int/life-course/about/who-health-innovation-group/en/. Accessed 28 May 2020.
-
- Office for Life Sciences and Welcome Trust. Accelerated Access Review: Final Report. 2016. https://wellcome.ac.uk/what-we-do/our-work/accelerated-access-review. Accessed 10 March 2017.
-
- Office for Life Sciences. Life Sciences Competitiveness Indicators. 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploa.... Accessed 29 May 2020.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources