Caspase-6 promotes activation of the caspase-11-NLRP3 inflammasome during gram-negative bacterial infections
- PMID: 34740613
- PMCID: PMC8633687
- DOI: 10.1016/j.jbc.2021.101379
Caspase-6 promotes activation of the caspase-11-NLRP3 inflammasome during gram-negative bacterial infections
Abstract
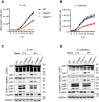
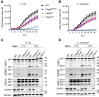
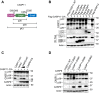
The innate immune system acts as the first line of defense against infection. One key component of the innate immune response to gram-negative bacterial infections is inflammasome activation. The caspase-11 (CASP11)-nucleotide-binding oligomerization domain-like receptor pyrin domain-containing 3 (NLRP3) inflammasome is activated by cytosolic lipopolysaccharide, a gram-negative bacterial cell wall component, to trigger pyroptosis and host defense during infection. Although several cellular signaling pathways have been shown to regulate CASP11-NLRP3 inflammasome activation in response to lipopolysaccharide, the upstream molecules regulating CASP11 activation during infection with live pathogens remain unclear. Here, we report that the understudied caspase-6 (CASP6) contributes to the activation of the CASP11-NLRP3 inflammasome in response to infections with gram-negative bacteria. Using in vitro cellular systems with bone marrow-derived macrophages and 293T cells, we found that CASP6 can directly process CASP11 by cleaving at Asp59 and Asp285, the CASP11 auto-cleavage sites, which could contribute to the activation of CASP11 during gram-negative bacterial infection. Thus, the loss of CASP6 led to impaired CASP11-NLRP3 inflammasome activation in response to gram-negative bacteria. These results demonstrate that CASP6 potentiates activation of the CASP11-NLRP3 inflammasome to produce inflammatory cytokines during gram-negative bacterial infections.
Keywords: C. rodentium; E. coli; NLRP3; P. aeruginosa; caspase-11; caspase-6; cell death; gasdermin D; gram-negative bacteria; inflammasome; pyroptosis.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Martinon F., Burns K., Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 2002;10:417–426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

