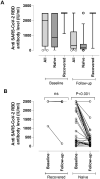
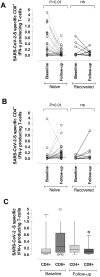
Evolution of SARS-CoV-2 immune responses in nursing home residents following full dose of the Comirnaty® COVID-19 vaccine
- PMID: 34740744
- PMCID: PMC8562042
- DOI: 10.1016/j.jinf.2021.10.026
Evolution of SARS-CoV-2 immune responses in nursing home residents following full dose of the Comirnaty® COVID-19 vaccine
Keywords: Comirnaty® COVID-19 vaccine; Nursing home residents; SARS-CoV-2; SARS-CoV-2-S T cells; SARS-CoV-2-S antibodies.
Conflict of interest statement
Declaration of Competing Interest The authors declare no conflicts of interest.
Figures
Comment on
-
Waning antibodies in SARS-CoV-2 naïve vaccinees: Results of a three-month interim analysis of ongoing immunogenicity and efficacy surveillance of the mRNA-1273 vaccine in healthcare workers.J Infect. 2021 Sep;83(3):381-412. doi: 10.1016/j.jinf.2021.06.017. Epub 2021 Jun 20. J Infect. 2021. PMID: 34161817 Free PMC article. No abstract available.
References
-
- Tré-Hardy M., Cupaiolo R., Wilmet A., Antoine-Moussiaux T., Della Vecchia A., Horeanga A., et al. Six-month interim analysis of ongoing immunogenicity surveillance of the mRNA-1273 vaccine in healthcare workers: a third dose is expected. J Infect. 2021;S0163–4453(21):00433. -3.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

