Timing of SARS-CoV-2 vaccination during the third trimester of pregnancy and transplacental antibody transfer: a prospective cohort study
- PMID: 34740773
- PMCID: PMC8563509
- DOI: 10.1016/j.cmi.2021.10.003
Timing of SARS-CoV-2 vaccination during the third trimester of pregnancy and transplacental antibody transfer: a prospective cohort study
Abstract
Objective: We aimed to assess the impact of early versus late third-trimester maternal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination on transplacental transfer and neonatal levels of SARS-CoV-2 antibodies.
Methods: Maternal and cord blood sera were collected following term delivery after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination, with the first vaccine dose administered between 27 and 36 weeks of gestation. SARS-CoV-2 spike protein (S) and receptor-binding domain (RBD) -specific, IgG levels and neutralizing potency were evaluated in maternal and cord blood samples.
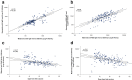
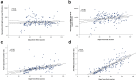
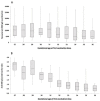
Results: The study cohort consisted of 171 parturients-median age 31 years (interquartile range (IQR) 27-35 years); median gestational age 39+5 weeks (IQR 38+5-40+4 weeks)-83 (48.5%) were immunized in early thrird-trimester (first dose at 27-31 weeks) and 88 (51.5%) were immunized in late third trimester (first dose at 32-36 weeks). All mother-infant paired sera were positive for anti S- and anti-RBD-specific IgG. Anti-RBD-specific IgG concentrations in neonatal sera were higher following early versus late third-trimester vaccination (median 9620 AU/mL (IQR 5131-15332 AU/mL) versus 6697 AU/mL (IQR 3157-14731 AU/mL), p 0.02), and were positively correlated with increasing time since vaccination (r = 0.26; p 0.001). Median antibody placental transfer ratios were increased following early versus late third-trimester immunization (anti-S ratio: 1.3 (IQR 1.1-1.6) versus 0.9 (IQR 0.6-1.1); anti-RBD-specific ratio: 2.3 (IQR 1.7-3.0) versus 0.7 (IQR 0.5-1.2), p < 0.001). Neutralizing antibodies placental transfer ratio was greater following early versus late third-trimester immunization (median 1.9 (IQR 1.7-2.5) versus 0.8 (IQR 0.5-1.1), p < 0.001), and was positively associated with longer duration from vaccination (r = 0.77; p < 0.001).
Conclusions: Early compared with late third-trimester maternal SARS-CoV-2 immunization enhanced transplacental antibody transfer and increased neonatal neutralizing antibody levels. Our findings highlight that vaccination of pregnant women early in the third trimester may enhance neonatal seroprotection.
Keywords: Cord blood; Coronavirus disease 2019; Passive immunity; Pregnancy; Serology; Severe acute respiratory syndrome coronavirus 2; Vaccination.
Copyright © 2021 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures

References
-
- Zambrano L.D., Ellington S., Strid P., Galang R.R., Oduyebo T., Tong V.T., et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641–1647. - PMC - PubMed
-
- Woodworth K.R., Olsen E.O., Neelam V., Lewis E.L., Galang R.R., Oduyebo T., et al. CDC COVID-19 response pregnancy and infant linked outcomes team; COVID-19 pregnancy and infant linked outcomes team (PILOT). Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy: SET-NET, 16 jurisdictions, March 29-october 14, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1635–1640. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

