Anterior pituitary, sex hormones, and keratoconus: Beyond traditional targets
- PMID: 34740824
- PMCID: PMC9058044
- DOI: 10.1016/j.preteyeres.2021.101016
Anterior pituitary, sex hormones, and keratoconus: Beyond traditional targets
Abstract
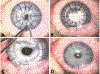
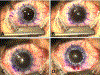
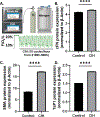
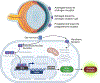
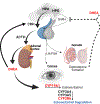
"The Diseases of the Horny-coat of The Eye", known today as keratoconus, is a progressive, multifactorial, non-inflammatory ectatic corneal disorder that is characterized by steepening (bulging) and thinning of the cornea, irregular astigmatism, myopia, and scarring that can cause devastating vision loss. The significant socioeconomic impact of the disease is immeasurable, as patients with keratoconus can have difficulties securing certain jobs or even joining the military. Despite the introduction of corneal crosslinking and improvements in scleral contact lens designs, corneal transplants remain the main surgical intervention for treating keratoconus refractory to medical therapy and visual rehabilitation. To-date, the etiology and pathogenesis of keratoconus remains unclear. Research studies have increased exponentially over the years, highlighting the clinical significance and international interest in this disease. Hormonal imbalances have been linked to keratoconus, both clinically and experimentally, with both sexes affected. However, it is unclear how (molecular/cellular signaling) or when (age/disease stage(s)) those hormones affect the keratoconic cornea. Previous studies have categorized the human cornea as an extragonadal tissue, showing modulation of the gonadotropins, specifically luteinizing hormone (LH) and follicle-stimulating hormone (FSH). Studies herein provide new data (both in vitro and in vivo) to further delineate the role of hormones/gonadotropins in the keratoconus pathobiology, and propose the existence of a new axis named the Hypothalamic-Pituitary-Adrenal-Corneal (HPAC) axis.
Keywords: Biomarkers; Eye; Gonadotropins; Keratoconus; Sex hormones.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interest
None
Figures


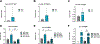




References
-
- Abad JC, Ocampo-Patino A, Vanegas-Ramirez C, 2020. Short-term bilateral keratoconus progression after deep anterior lamellar keratoplasty in one eye and intracorneal ring segments and corneal crosslinking in the other due to eye rubbing. J Cataract Refract Surg 46, e44–e47. - PubMed
-
- Abboud EB, 1998. Retinal detachment surgery in Marfan’s syndrome. Retina 18, 405–409. - PubMed
-
- Affinito P, Di Spiezio Sardo A, Di Carlo C, Sammartino A, Tommaselli GA, Bifulco G, Loffredo A, Loffredo M, Nappi C, 2003. Effects of hormone replacement therapy on ocular function in postmenopause. Menopause 10, 482–487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

