Diminished Efficacy of Programmed Death-(Ligand)1 Inhibition in STK11- and KEAP1-Mutant Lung Adenocarcinoma Is Affected by KRAS Mutation Status
- PMID: 34740862
- PMCID: PMC10980559
- DOI: 10.1016/j.jtho.2021.10.013
Diminished Efficacy of Programmed Death-(Ligand)1 Inhibition in STK11- and KEAP1-Mutant Lung Adenocarcinoma Is Affected by KRAS Mutation Status
Abstract
Introduction: STK11 and KEAP1 mutations (STK11 mutant [STK11MUT] and KEAP1MUT) are among the most often mutated genes in lung adenocarcinoma (LUAD). Although STK11MUT has been associated with resistance to programmed death-(ligand)1 (PD-[L]1) inhibition in KRASMUT LUAD, its impact on immunotherapy efficacy in KRAS wild-type (KRASWT) LUAD is currently unknown. Whether KEAP1MUT differentially affects outcomes to PD-(L)1 inhibition in KRASMUT and KRASWT LUAD is also unknown.
Methods: Clinicopathologic and genomic data were collected from September 2013 to September 2020 from patients with advanced LUAD at the Dana-Farber Cancer Institute/Massachusetts General Hospital cohort and the Memorial Sloan Kettering Cancer Center/MD Anderson Cancer Center cohort. Clinical outcomes to PD-(L)1 inhibition were analyzed according to KRAS, STK11, and KEAP1 mutation status in two independent cohorts. The Cancer Genome Atlas transcriptomic data were interrogated to identify differences in tumor gene expression and tumor immune cell subsets, respectively, according to KRAS/STK11 and KRAS/KEAP1 comutation status.
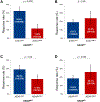
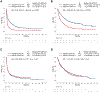
Results: In the combined cohort (Dana-Farber Cancer Institute/Massachusetts General Hospital + Memorial Sloan Kettering Cancer Center/MD Anderson Cancer Center) of 1261 patients (median age = 61 y [range: 22-92], 708 women [56.1%], 1065 smokers [84.4%]), KRAS mutations were detected in 536 cases (42.5%), and deleterious STK11 and KEAP1 mutations were found in 20.6% (260 of 1261) and 19.2% (231 of 1202) of assessable cases, respectively. In each independent cohort and in the combined cohort, STK11 and KEAP1 mutations were associated with significantly worse progression-free (STK11 hazard ratio [HR] = 2.04, p < 0.0001; KEAP1 HR = 2.05, p < 0.0001) and overall (STK11 HR = 2.09, p < 0.0001; KEAP1 HR = 2.24, p < 0.0001) survival to immunotherapy uniquely among KRASMUT but not KRASWT LUADs. Gene expression ontology and immune cell enrichment analyses revealed that the presence of STK11 or KEAP1 mutations results in distinct immunophenotypes in KRASMUT, but not in KRASWT, lung cancers.
Conclusions: STK11 and KEAP1 mutations confer worse outcomes to immunotherapy among patients with KRASMUT but not among KRASWT LUAD. Tumors harboring concurrent KRAS/STK11 and KRAS/KEAP1 mutations display distinct immune profiles in terms of gene expression and immune cell infiltration.
Keywords: KEAP1; KRAS; NSCLC; PD-(L)1 blockade; STK11.
Copyright © 2021 International Association for the Study of Lung Cancer. Published by Elsevier Inc. All rights reserved.
Figures




Comment in
-
STK11 and KEAP1 Mutations in Lung Adenocarcinoma: Solving the Puzzle Continues.J Thorac Oncol. 2022 Mar;17(3):351-352. doi: 10.1016/j.jtho.2022.01.004. J Thorac Oncol. 2022. PMID: 35216730 No abstract available.
-
Letter to the Editor Concerning Diminished Efficacy of Programmed Death-(Ligand) 1 Inhibition in STK11- and KEAP1-Mutant Lung Adenocarcinoma Is Affected by KRAS Mutation Status.J Thorac Oncol. 2022 Jun;17(6):e63-e64. doi: 10.1016/j.jtho.2022.01.022. J Thorac Oncol. 2022. PMID: 35623683 No abstract available.
-
Reply to Kus and Aktas.J Thorac Oncol. 2022 Jun;17(6):e64-e65. doi: 10.1016/j.jtho.2022.04.004. J Thorac Oncol. 2022. PMID: 35623684 No abstract available.
References
-
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. - PubMed
-
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. - PubMed
-
- Aguilar EJ, Ricciuti B, Gainor JF, et al. Outcomes to first-line pembrolizumab in patients with non-small-cell lung cancer and very high PD-L1 expression. Ann Oncol. 2019;30:1653–1659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

