Multi-omics of human plasma reveals molecular features of dysregulated inflammation and accelerated aging in schizophrenia
- PMID: 34741130
- PMCID: PMC9054664
- DOI: 10.1038/s41380-021-01339-z
Multi-omics of human plasma reveals molecular features of dysregulated inflammation and accelerated aging in schizophrenia
Abstract
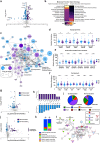
Schizophrenia is a devastating psychiatric illness that detrimentally affects a significant portion of the worldwide population. Aging of schizophrenia patients is associated with reduced longevity, but the potential biological factors associated with aging in this population have not yet been investigated in a global manner. To address this gap in knowledge, the present study assesses proteomics and metabolomics profiles in the plasma of subjects afflicted with schizophrenia compared to non-psychiatric control patients over six decades of life. Global, unbiased analyses of circulating blood plasma can provide knowledge of prominently dysregulated molecular pathways and their association with schizophrenia, as well as features of aging and gender in this disease. The resulting data compiled in this study represent a compendium of molecular changes associated with schizophrenia over the human lifetime. Supporting the clinical finding of schizophrenia's association with more rapid aging, both schizophrenia diagnosis and age significantly influenced the plasma proteome in subjects assayed. Schizophrenia was broadly associated with prominent dysregulation of inflammatory and metabolic system components. Proteome changes demonstrated increased abundance of biomarkers for risk of physiologic comorbidities of schizophrenia, especially in younger individuals. These findings advance our understanding of the molecular etiology of schizophrenia and its associated comorbidities throughout the aging process.
© 2021. The Author(s).
Conflict of interest statement
PD is a scientific advisor to Sirenas, Cybele and Galileo and Co-founder and scientific advisor to Ometa and Enveda. All other authors declare no competing interests.
Figures



References
-
- Kilbourne AM, Morden NE, Austin K, Ilgen M, McCarthy JF, Dalack G, et al. Excess heart-disease-related mortality in a national study of patients with mental disorders: identifying modifiable risk factors. Gen Hosp Psychiatry. 2009;31:555–63. doi: 10.1016/j.genhosppsych.2009.07.008. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

