Comprehensive Review of Uterine Fibroids: Developmental Origin, Pathogenesis, and Treatment
- PMID: 34741454
- PMCID: PMC9277653
- DOI: 10.1210/endrev/bnab039
Comprehensive Review of Uterine Fibroids: Developmental Origin, Pathogenesis, and Treatment
Erratum in
-
Corrigendum to: Comprehensive Review of Uterine Fibroids: Developmental Origin, Pathogenesis, and Treatment.Endocr Rev. 2022 Jul 13;43(4):761. doi: 10.1210/endrev/bnac007. Endocr Rev. 2022. PMID: 35234847 Free PMC article. No abstract available.
-
Erratum to: Comprehensive Review of Uterine Fibroids: Developmental Origin, Pathogenesis, and Treatment.Endocr Rev. 2022 Jul 13;43(4):762. doi: 10.1210/endrev/bnac006. Endocr Rev. 2022. PMID: 35234873 Free PMC article. No abstract available.
Abstract
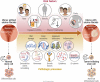
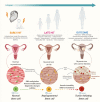
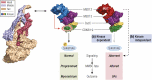
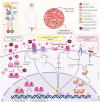
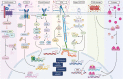
Uterine fibroids are benign monoclonal neoplasms of the myometrium, representing the most common tumors in women worldwide. To date, no long-term or noninvasive treatment option exists for hormone-dependent uterine fibroids, due to the limited knowledge about the molecular mechanisms underlying the initiation and development of uterine fibroids. This paper comprehensively summarizes the recent research advances on uterine fibroids, focusing on risk factors, development origin, pathogenetic mechanisms, and treatment options. Additionally, we describe the current treatment interventions for uterine fibroids. Finally, future perspectives on uterine fibroids studies are summarized. Deeper mechanistic insights into tumor etiology and the complexity of uterine fibroids can contribute to the progress of newer targeted therapies.
Keywords: developmental origin; epigenetics pathways; future directions; genetic instability; novel treatment; reprogramming; uterine fibroids.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Endocrine Society.
Figures


References
-
- Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100-107. - PubMed
-
- Stewart EA, Laughlin-Tommaso SK, Catherino WH, Lalitkumar S, Gupta D, Vollenhoven B. Uterine fibroids. Nat Rev Dis Primers. 2016;2:16043. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

