Neocortex saves energy by reducing coding precision during food scarcity
- PMID: 34741806
- PMCID: PMC8788933
- DOI: 10.1016/j.neuron.2021.10.024
Neocortex saves energy by reducing coding precision during food scarcity
Abstract
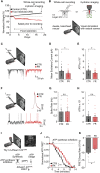
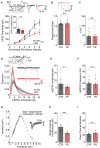
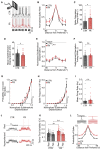
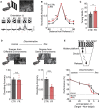
Information processing is energetically expensive. In the mammalian brain, it is unclear how information coding and energy use are regulated during food scarcity. Using whole-cell recordings and two-photon imaging in layer 2/3 mouse visual cortex, we found that food restriction reduced AMPA receptor conductance, reducing synaptic ATP use by 29%. Neuronal excitability was nonetheless preserved by a compensatory increase in input resistance and a depolarized resting potential. Consequently, neurons spiked at similar rates as controls but spent less ATP on underlying excitatory currents. This energy-saving strategy had a cost because it amplified the variability of visually-evoked subthreshold responses, leading to a 32% broadening of orientation tuning and impaired fine visual discrimination. This reduction in coding precision was associated with reduced levels of the fat mass-regulated hormone leptin and was restored by exogenous leptin supplementation. Our findings reveal that metabolic state dynamically regulates the energy spent on coding precision in neocortex.
Keywords: calorie restriction; hunger and satiety; in vivo ATP imaging; in vivo calcium imaging; in vivo electrophysiology; leptin; mouse primary visual cortex; orientation tuning; spike rate homeostasis; trial-to-trial variability.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests
Figures



Comment in
-
More than meets the eye: The metabolic state of the body shapes visual sensations.Cell Metab. 2022 Jan 4;34(1):9-10. doi: 10.1016/j.cmet.2021.12.006. Cell Metab. 2022. PMID: 34986340
-
Metabolic demands, sensory deficits: Tradeoffs in times of scarcity.Neuron. 2022 Jan 19;110(2):183-184. doi: 10.1016/j.neuron.2021.12.011. Neuron. 2022. PMID: 35051362 Free PMC article.
References
-
- Ahima R.S., Prabakaran D., Mantzoros C., Qu D., Lowell B., Maratos-Flier E., Flier J.S. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. - PubMed
-
- Arieli A., Sterkin A., Grinvald A., Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. - PubMed
-
- Attwell D., Laughlin S.B. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 2001;21:1133–1145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

