NF1-mutated melanomas reveal distinct clinical characteristics depending on tumour origin and respond favourably to immune checkpoint inhibitors
- PMID: 34742158
- PMCID: PMC9431958
- DOI: 10.1016/j.ejca.2021.09.035
NF1-mutated melanomas reveal distinct clinical characteristics depending on tumour origin and respond favourably to immune checkpoint inhibitors
Abstract
Background: NF1-mutated tumours represent a small subset (10-15%) of melanomas, not sufficiently analysed in large clinical cohorts. This study investigated the largest multicentre collection of NF1-mutated melanomas to date.
Methods: This study analysed a multicentre tumour tissue sample cohort from 266 patients with NF1-mutated melanoma. Targeted next-generation sequencing of the TERT promoter and 29 relevant melanoma genes was performed. Survival was compared with NF1 wild-type cohorts from the Tissue Registry in Melanoma project (n = 432).
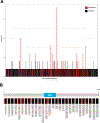
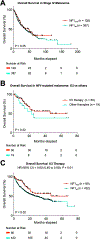
Results: Most NF1-mutated melanoma arose in the head-and-neck region of patients >60 years. NF1 alterations were frequently inactivating, primarily non-sense, less frequently truncating mutations. Non-inactivating NF1 mutations more frequently co-occurred with activating BRAF and RAS mutations. NF1-mutated tumours had higher numbers of gene mutations and UV signature C>T and CC>TT transitions than BRAF, RAS and triple wild-type melanomas. NF1-mutated acral and mucosal melanomas harboured a different mutation signature and were frequent in women (69% and 83%, respectively), differing from non-acral cutaneous NF1-mutated melanomas (men 73%, women 27%). Overall survival in stage IV disease was comparable for patients with NF1-mutated or wild-type melanoma. However, in patients receiving first-line immune checkpoint inhibitor treatment, better median overall survival (mOS) was observed for NF1-mutated than wild-type tumours (mOS = not reached vs mOS = 25.82, p = 0.0154, n = 80 and 432, respectively).
Conclusions: Cutaneous, acral and mucosal NF1-mutated melanomas vary in clinical and genetic characteristics and demonstrate a favourable outcome on immune checkpoint inhibition therapy.
Keywords: BRAF; Melanoma; Mutation profiling; NF1; NRAS.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
C.M.T.: No relevant conflicts of interest.
E.C.: No relevant conflicts of interest.
J.M.: Declares travel support from Bristol Myers Squibb, Novartis and Sun Pharmaceutical Industries, outside the submitted work.
R.M.: No relevant conflicts of interest.
A.Z.: Declares travel support from Novartis, Sanofi Grenzyme, and Bristol-Myers Squibb, outside the submitted work.
G.L.: Declares travel support from Sun Pharma, outside the submitted work.
P.J.: No relevant conflicts of interest.
L.R.: No relevant conflicts of interest.
J.K.: No relevant conflicts of interest.
I.M.: No relevant conflicts of interest.
A.S.: No relevant conflicts of interest.
R.H.: Declares speakers and advisory board honoraria from BMS, MSD, Novartis, Pierre Fabre, Roche and SUN-Pharma, outside the submitted work.
P.T.: Declares honoraria from Bristol-Myers Squibb, Novartis, Merck Sharp & Dohme, Pierre-Fabre, CureVac, Merck Serono, Sanofi, Roche, Kyowa Kirin, Biofrontera; travel support from Bristol-Myers Squibb and Pierre-Fabre, outside the submitted work.
J.U.: Declares advisory board or has received honoraria and travel support from Amgen, Bristol Myers Squibb, GSK, LeoPharma, Merck Sharp and Dohme, Novartis, Pierre Fabre, Roche, Sanofi, outside the submitted work.
C.P.: served as consultant and/or has received honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Sanofi, Sunpharma, Pierre Fabre, AbbVie, Kyona Kirin and Amgen and received travel support from Amgen, Merck Sharp & Dohme, Bristol-Myers Squibb, Pierre Fabre, Sunpharma and Novartis, outside the submitted work.
J.Ul: Declares travel support: Medac, Sun Pharma; consulting: Bristol-MyersSquibb, Sun Pharma; lectures: Bristol-MyersSquibb, MSD, Merck, Novartis, Roche, Sanofi, Sun Pharma; grants: Novartis, outside the submitted work.
A.K.: No relevant conflicts of interest.
P.M.: Declares research support (to institution): Bristol-MyersSquibb, Novartis, MSD. Honoraria for lectures (personally): Roche Pharma, Bristol-MyersSquibb, Novartis, MSD, Almirall-Hermal, Amgen, Merck-Serono, Bayer, Pierre-Fabre, Sanofi. Honoraria for advisory boards: Bayersdorf, Roche Pharma, Bristol-MyersSquibb, Novartis, MSD, Almirall-Hermal, Amgen, Pierre-Fabre, Merck-Serono, SUN, Merck-Serono, Sanofi, outside the submitted work.
R.G.: Invited speaker: Roche, BMS, MSD, Novartis, Amgen, Merck Serono, Almirall Hermal, SUN, Sanofi, Pierre-Fabre. Advisory board: BMS, Roche, Novartis, Almirall Hermal, MSD, Amgen, SUN, Sanofi, Pierre-Fabre, 4SC, Bayer, MerckSerono, Pfizer, Immunocore. Research grants: Novartis, Pfizer, Johnson & Johnson, Amgen, Merck-Serono, SUN Pharma, Sanofi. Travel/meeting support: Roche, BMS, SUN, Merck-Serono, Pierre-Fabre, outside the submitted work.
F.M.: Declares travel support or/and speaker’s fees or/and advisor’s honoraria by Novartis, Roche, BMS, MSD and Pierre Fabre and research funding from Novartis and Roche, outside the submitted work.
E.D.: No relevant conflicts of interest.
M.W.: No relevant conflicts of interest.
A.P.: No relevant conflicts of interest.
E.L.: Served as consultant and/or has received honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Medac, Sanofi, Sunpharma and travel support from Amgen, Merck Sharp & Dohme, Bristol-Myers Squibb, Pierre Fabre, Sunpharma and Novartis, outside the submitted work.
LZ: served as consultant and/or has received honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Pierre-Fabre, Sunpharma and Sanofi; Research funding to institution: Novartis; travel support from Merck Sharp & Dohme, Bristol-Myers Squibb, Amgen, Pierre-Fabre, Sanofi, Sunpharma and Novartis, outside the submitted work.
D.S. reports personal fees and non-financial support from Roche/Genentech, grants, personal fees, non-financial support and other from BMS, personal fees from Merck Sharp & Dohme, personal fees and non-financial support from Merck Serono, grant, personal fees and non-financial support from Amgen, personal fees from Immunocore, personal fees from Incyte, personal fees from 4SC, personal fees from Pierre Fabre, personal fees and non-financial support from Sanofi/Regeneron, personal fees from Array BioPharma, personal fees from Pfizer, personal fees from Philogen, personal fees from Regeneron, personal fees from Nektar, personal fees from Sandoz, grants, personal fees and non-financial support from Novartis, personal fees and non-financial support from SunPharma, Replimune, Helsinn, OncoSec and InFlaRx outside the submitted work.
E.H.: No relevant conflicts of interest.
S.U. declares research support from Bristol Myers Squibb and Merck Serono; speakers and advisory board honoraria from Bristol Myers Squibb, Merck Sharp & Dohme, Merck Serono, Novartis and Roche, and travel support from Bristol Myers Squibb, and Merck Sharp & Dohme, outside the submitted work.
K.G.: No relevant conflicts of interest.
Figures


References
-
- Ajithkumar T, Parkinson C, Fife K, Corrie P, Jefferies S. Evolving treatment options for melanoma brain metastases. The lancet oncology. 2015;16(13):e486–497. - PubMed
-
- Schadendorf D, van Akkooi ACJ, Berking C, et al. Melanoma. Lancet. 2018;392(10151):971–984. - PubMed
-
- Ugurel S, Rohmel J, Ascierto PA, et al. Survival of patients with advanced metastatic melanoma: The impact of MAP kinase pathway inhibition and immune checkpoint inhibition - Update 2019. Eur J Cancer. 2020;130:126–138. - PubMed
-
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. The New England journal of medicine. 2005;353(20):2135–2147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

