Harms in Systematic Reviews Paper 1: An introduction to research on harms
- PMID: 34742788
- PMCID: PMC9126149
- DOI: 10.1016/j.jclinepi.2021.10.023
Harms in Systematic Reviews Paper 1: An introduction to research on harms
Abstract
Objective: Most systematic reviews of interventions focus on potential benefits. Common methods and assumptions that are appropriate for assessing benefits can be inappropriate for harms. This paper provides a primer on researching harms, particularly in systematic reviews.
Study design and setting: Commentary describing challenges with assessing harm.
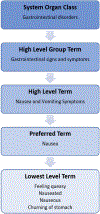
Results: Investigators should be familiar with various terminologies used to describe, classify, and group harms. Published reports of clinical trials include limited information about harms, so systematic reviewers should not depend on these studies and journal articles to reach conclusions about harms. Visualizations might improve communication of multiple dimensions of harms such as severity, relatedness, and timing.
Conclusion: The terminology, classification, detection, collection, and reporting of harms create unique challenges that take time, expertise, and resources to navigate in both primary studies and evidence syntheses. Systematic reviewers might reach incorrect conclusions if they focus on evidence about harms found in published reports of randomized trials of a particular health problem. Systematic reviews could be improved through better identification and reporting of harms in primary studies and through better training and uptake of appropriate methods for synthesizing evidence about harms.
Keywords: Clinical Trials; Harms; Meta-analysis; Synthesis; Systematic Reviews.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Conflict of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources