Harms in Systematic Reviews Paper 3: Given the same data sources, systematic reviews of gabapentin have different results for harms
- PMID: 34742790
- PMCID: PMC9875741
- DOI: 10.1016/j.jclinepi.2021.10.025
Harms in Systematic Reviews Paper 3: Given the same data sources, systematic reviews of gabapentin have different results for harms
Abstract
Objective: In this methodologic study (Part 2 of 2), we examined the overlap in sources of evidence and the corresponding results for harms in systematic reviews for gabapentin.
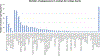
Study design & setting: We extracted all citations referenced as sources of evidence for harms of gabapentin from 70 systematic reviews, as well as the harms assessed and numerical results. We assessed consistency of harms between pairs of reviews with a high degree of overlap in sources of evidence (>50%) as determined by corrected covered area (CCA).
Results: We found 514 reports cited across 70 included reviews. Most reports (244/514, 48%) were not cited in more than one review. Among 18 pairs of reviews, we found reviews had differences in which harms were assessed and their choice to meta-analyze estimates or present descriptive summaries. When a specific harm was meta-analyzed in a pair of reviews, we found similar effect estimates.
Conclusion: Differences in harms results across reviews can occur because the choice of harms is driven by reviewer preferences, rather than standardized approaches to selecting harms for assessment. A paradigm shift is needed in the current approach to synthesizing harms.
Keywords: Clinical Trials; Harms; Meta-analysis; Synthesis; Systematic Reviews.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Peryer G, Golder S, Junqueira D, Vohra S, Kong Loke Y, et al. Chapter 19: Adverse effects Cochrane Handbook for Systematic Reviews of Interventions. Version 6. Cochrane. Higgins J, Thomas J, Chandler J, et al., editors; 2019. https://training.cochrane.org/handbook/version-6/chapter-19-draftv2.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources