Management of altered metabolic activity in Drosophila model of Huntington's disease by curcumin
- PMID: 34743577
- PMCID: PMC8777478
- DOI: 10.1177/15353702211046927
Management of altered metabolic activity in Drosophila model of Huntington's disease by curcumin
Abstract
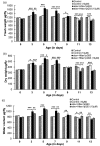
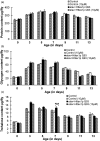
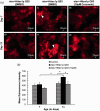
Huntington's disease (HD) is a devastating polyglutamine disorder characterized by extensive neurodegeneration and metabolic abnormalities at systemic, cellular and intracellular levels. Metabolic alterations in HD manifest as abnormal body weight, dysregulated biomolecule levels, impaired adipocyte functions, and defective energy state which exacerbate disease progression and pose acute threat to the health of challenged individuals in form of insulin resistance, cardiovascular disease, and energy crisis. To colossally mitigate disease symptoms, we tested the efficacy of curcumin in Drosophila model of HD. Curcumin is the bioactive component of turmeric (Curcuma longa Linn), well-known for its ability to modulate metabolic activities. We found that curcumin effectively managed abnormal body weight, dysregulated lipid content, and carbohydrate level in HD flies. In addition, curcumin administration lowered elevated reactive-oxygen-species levels in adult adipose tissue of diseased flies, and improved survival and locomotor function in HD flies at advanced disease stage. Altogether, these findings clearly suggest that curcumin efficiently attenuates metabolic derangements in HD flies and can prove beneficial in alleviating the complexities associated with HD.
Keywords: Body weight; Huntington’s disease; curcumin; lipid metabolism; neurodegenerative disease; reactive-oxygen-species.
Conflict of interest statement
Figures




References
-
- The Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosome. Cell 1993; 72:971–83 - PubMed
-
- Sanberg PR, Fibiger HC, Mark RF. Body weight and dietary factors in Huntington’s disease patients compared with matched controls. Med J Aust 1981; 1:407–9 - PubMed
-
- Gines S, Seong IS, Fossale E, Ivanova E, Trettel F, Gusella JF, Wheeler VC, Persichetti F, MacDonald ME. Specific progressive cAMP reduction implicates energy deficit in presymptomatic Huntington's disease knock-in mice. Hum Mol Genet 2003; 12:497–508 - PubMed
-
- Björkqvist M, Petersén A, Bacos K, Isaacs J, Norlén P, Gil J, Popovic N, Sundler F, Bates GP, Tabrizi SJ, Brundin P, Mulder H. Progressive alterations in the hypothalamic-pituitary-adrenal axis in the R6/2 transgenic mouse model of Huntington’s disease. Hum Mol Genet 2006; 15:1713–21 - PubMed
-
- Popovic V, Svetel M, Djurovic M, Petrovic S, Doknic M, Pekic S, Miljic D, Milic N, Glodic J, Dieguez C, Casanueva FF, Kostic V. Circulating and cerebrospinal fluid ghrelin and leptin: potential role in altered body weight in Huntington’s disease. Eur J Endocrinol 2004; 151:451–5 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

