Targeting lysosomes in human disease: from basic research to clinical applications
- PMID: 34744168
- PMCID: PMC8572923
- DOI: 10.1038/s41392-021-00778-y
Targeting lysosomes in human disease: from basic research to clinical applications
Abstract
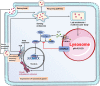
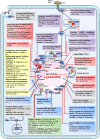
In recent years, accumulating evidence has elucidated the role of lysosomes in dynamically regulating cellular and organismal homeostasis. Lysosomal changes and dysfunction have been correlated with the development of numerous diseases. In this review, we interpreted the key biological functions of lysosomes in four areas: cellular metabolism, cell proliferation and differentiation, immunity, and cell death. More importantly, we actively sought to determine the characteristic changes and dysfunction of lysosomes in cells affected by these diseases, the causes of these changes and dysfunction, and their significance to the development and treatment of human disease. Furthermore, we outlined currently available targeting strategies: (1) targeting lysosomal acidification; (2) targeting lysosomal cathepsins; (3) targeting lysosomal membrane permeability and integrity; (4) targeting lysosomal calcium signaling; (5) targeting mTOR signaling; and (6) emerging potential targeting strategies. Moreover, we systematically summarized the corresponding drugs and their application in clinical trials. By integrating basic research with clinical findings, we discussed the current opportunities and challenges of targeting lysosomes in human disease.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

