The Immediate and Short-Term Effects of Transcutaneous Spinal Cord Stimulation and Peripheral Nerve Stimulation on Corticospinal Excitability
- PMID: 34744614
- PMCID: PMC8566815
- DOI: 10.3389/fnins.2021.749042
The Immediate and Short-Term Effects of Transcutaneous Spinal Cord Stimulation and Peripheral Nerve Stimulation on Corticospinal Excitability
Abstract
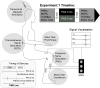
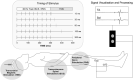
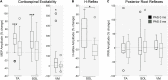
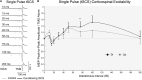
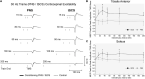
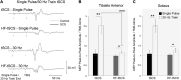
Rehabilitative interventions involving electrical stimulation show promise for neuroplastic recovery in people living with Spinal Cord Injury (SCI). However, the understanding of how stimulation interacts with descending and spinal excitability remain unclear. In this study we compared the immediate and short-term (within a few minutes) effects of pairing Transcranial Magnetic Stimulation (TMS) with transcutaneous Spinal Cord stimulation (tSCS) and Peripheral Nerve Stimulation (PNS) on Corticospinal excitability in healthy subjects. Three separate experimental conditions were assessed. In Experiment I, paired associative stimulation (PAS) was applied, involving repeated pairing of single pulses of TMS and tSCS, either arriving simultaneously at the spinal motoneurones (PAS0ms) or slightly delayed (PAS5ms). Corticospinal and spinal excitability, and motor performance, were assessed before and after the PAS interventions in 24 subjects. Experiment II compared the immediate effects of tSCS and PNS on corticospinal excitability in 20 subjects. Experiment III compared the immediate effects of tSCS with tSCS delivered at the same stimulation amplitude but modulated with a carrier frequency (in the kHz range) on corticospinal excitability in 10 subjects. Electromyography (EMG) electrodes were placed over the Tibialis Anterior (TA) soleus (SOL) and vastus medialis (VM) muscles and stimulation electrodes (cathodes) were placed on the lumbar spine (tSCS) and lateral to the popliteal fossa (PNS). TMS over the primary motor cortex (M1) was paired with tSCS or PNS to produce Motor Evoked Potentials (MEPs) in the TA and SOL muscles. Simultaneous delivery of repetitive PAS (PAS0ms) increased corticospinal excitability and H-reflex amplitude at least 5 min after the intervention, and dorsiflexion force was increased in a force-matching task. When comparing effects on descending excitability between tSCS and PNS, a subsequent facilitation in MEPs was observed following tSCS at 30-50 ms which was not present following PNS. To a lesser extent this facilitatory effect was also observed with HF- tSCS at subthreshold currents. Here we have shown that repeated pairing of TMS and tSCS can increase corticospinal excitability when timed to arrive simultaneously at the alpha-motoneurone and can influence functional motor output. These results may be useful in optimizing stimulation parameters for neuroplasticity in people living with SCI.
Keywords: corticospinal excitability; paired associative stimulation (PAS); peripheral nerve stimulation (PNS); rehabilitation; spinal cord stimulation (SCS); transcranial magnetic stimulation.
Copyright © 2021 Al’joboori, Hannah, Lenham, Borgas, Kremers, Bunday, Rothwell and Duffell.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

