The Large-Conductance, Calcium-Activated Potassium Channel: A Big Key Regulator of Cell Physiology
- PMID: 34744788
- PMCID: PMC8567177
- DOI: 10.3389/fphys.2021.750615
The Large-Conductance, Calcium-Activated Potassium Channel: A Big Key Regulator of Cell Physiology
Abstract
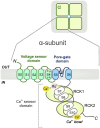
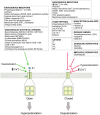
Large-conductance Ca2+-activated K+ channels facilitate the efflux of K+ ions from a variety of cells and tissues following channel activation. It is now recognized that BK channels undergo a wide range of pre- and post-translational modifications that can dramatically alter their properties and function. This has downstream consequences in affecting cell and tissue excitability, and therefore, function. While finding the "silver bullet" in terms of clinical therapy has remained elusive, ongoing research is providing an impressive range of viable candidate proteins and mechanisms that associate with and modulate BK channel activity, respectively. Here, we provide the hallmarks of BK channel structure and function generally, and discuss important milestones in the efforts to further elucidate the diverse properties of BK channels in its many forms.
Keywords: BK channels; intracellular Ca2+; membrane potential; nervous system; smooth muscle.
Copyright © 2021 Sancho and Kyle.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Baylie R., Ahmed M., Bonev A. D., Hill-Eubanks D. C., Heppner T. J., Nelson M. T., et al. . (2017). Lack of direct effect of adiponectin on vascular smooth muscle cell BKCa channels or Ca2+ signaling in the regulation of small artery pressure-induced constriction. Physiol. Rep. 5:e13337. doi: 10.14814/phy2.13337 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

