Microbiome-Specific Statistical Modeling Identifies Interplay Between Gastrointestinal Microbiome and Neurobehavioral Outcomes in Patients With Autism: A Case Control Study
- PMID: 34744810
- PMCID: PMC8563626
- DOI: 10.3389/fpsyt.2021.682454
Microbiome-Specific Statistical Modeling Identifies Interplay Between Gastrointestinal Microbiome and Neurobehavioral Outcomes in Patients With Autism: A Case Control Study
Abstract
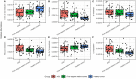
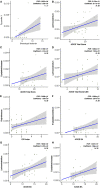
Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder with unclear mechanisms of pathogenesis. Gastrointestinal microbiome alterations were found to correlate with ASD core symptoms, but its specific role in ASD pathogenesis has not been determined. In this study, we used a case-control strategy that simultaneously compared the ASD gastrointestinal microbiome with that from age-sex matched controls and first-degree relative controls, using a statistical framework accounting for confounders such as age. Enterobacteriaceae (including Escherichia/Shigella) and Phyllobacterium were significantly enriched in the ASD group, with their relative abundances all following a pattern of ASD > first degree relative control > healthy control, consistent with our hypothesis of living environment and shared microbial and immunological exposures as key drivers of ASD gastrointestinal microbiome dysbiosis. Using multivariable omnibus testing, we identified clinical factors including ADOS scores, dietary habits, and gastrointestinal symptoms that covary with overall microbiome structure within the ASD cohort. A microbiome-specific multivariate modeling approach (MaAsLin2) demonstrated microbial taxa, such as Lachnoclostridium and Tyzzerella, are significantly associated with ASD core symptoms measured by ADOS. Finally, we identified alterations in predicted biological functions, including tryptophan and tyrosine biosynthesis/metabolism potentially relevant to the pathophysiology of the gut-brain-axis. Overall, our results identified gastrointestinal microbiome signature changes in patients with ASD, highlighted associations between gastrointestinal microbiome and clinical characteristics related to the gut-brain axis and identified contributors to the heterogeneity of gastrointestinal microbiome within the ASD population.
Keywords: autism spectrum disorder; biological pathway; gut microbiome; gut-brain axis; multivariable omnibus testing; predictive functional profiling.
Copyright © 2021 Huang, Liu, Liu, Chen, Wei, Feng, Wu, Fong, Tian, Wang, Budjan, Zhuang, Wan and Kong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
New and Preliminary Evidence on Altered Oral and Gut Microbiota in Individuals with Autism Spectrum Disorder (ASD): Implications for ASD Diagnosis and Subtyping Based on Microbial Biomarkers.Nutrients. 2019 Sep 6;11(9):2128. doi: 10.3390/nu11092128. Nutrients. 2019. PMID: 31489949 Free PMC article.
-
Microbiome-Gut-Mucosal-Immune-Brain Axis and Autism Spectrum Disorder (ASD): A Novel Proposal of the Role of the Gut Microbiome in ASD Aetiology.Behav Sci (Basel). 2023 Jun 30;13(7):548. doi: 10.3390/bs13070548. Behav Sci (Basel). 2023. PMID: 37503995 Free PMC article. Review.
-
Gut Microbial Dysbiosis in Indian Children with Autism Spectrum Disorders.Microb Ecol. 2018 Nov;76(4):1102-1114. doi: 10.1007/s00248-018-1176-2. Epub 2018 Mar 21. Microb Ecol. 2018. PMID: 29564487
-
Altered Autonomic Functions and Gut Microbiome in Individuals with Autism Spectrum Disorder (ASD): Implications for Assisting ASD Screening and Diagnosis.J Autism Dev Disord. 2021 Jan;51(1):144-157. doi: 10.1007/s10803-020-04524-1. J Autism Dev Disord. 2021. PMID: 32410097
-
Intestinal Barrier Dysfunction and Microbiota-Gut-Brain Axis: Possible Implications in the Pathogenesis and Treatment of Autism Spectrum Disorder.Nutrients. 2023 Mar 27;15(7):1620. doi: 10.3390/nu15071620. Nutrients. 2023. PMID: 37049461 Free PMC article. Review.
Cited by
-
Deficient butyrate-producing capacity in the gut microbiome is associated with bacterial network disturbances and fatigue symptoms in ME/CFS.Cell Host Microbe. 2023 Feb 8;31(2):288-304.e8. doi: 10.1016/j.chom.2023.01.004. Cell Host Microbe. 2023. PMID: 36758522 Free PMC article.
-
Autism spectrum disorders and the gastrointestinal tract: insights into mechanisms and clinical relevance.Nat Rev Gastroenterol Hepatol. 2024 Mar;21(3):142-163. doi: 10.1038/s41575-023-00857-1. Epub 2023 Dec 19. Nat Rev Gastroenterol Hepatol. 2024. PMID: 38114585 Review.
-
A systematic review on the impact of gastrointestinal microbiota composition and function on cognition in healthy infants and children.Front Neurosci. 2023 Jun 14;17:1171970. doi: 10.3389/fnins.2023.1171970. eCollection 2023. Front Neurosci. 2023. PMID: 37389363 Free PMC article. Review.
-
Overall Rebalancing of Gut Microbiota Is Key to Autism Intervention.Front Psychol. 2022 May 26;13:862719. doi: 10.3389/fpsyg.2022.862719. eCollection 2022. Front Psychol. 2022. PMID: 35712154 Free PMC article. Review.
-
Neurodevelopmental Disorders Associated with Gut Microbiome Dysbiosis in Children.Children (Basel). 2024 Jun 28;11(7):796. doi: 10.3390/children11070796. Children (Basel). 2024. PMID: 39062245 Free PMC article. Review.
References
-
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. . Prevalence of autism spectrum disorder among children aged 8 years — autism and developmental disabilities monitoring network, 11 sites, United States, 2014. Mmwr Surveillance Summ. (2018) 67:1–23. 10.15585/mmwr.ss6706a1 - DOI - PMC - PubMed
-
- Liu J, Zhang M, Kong X-J. Gut microbiome and autism: recent advances and future perspectives. N A J Med Sci. (2016) 9:104–115. 10.7156/najms.2016.0903104 - DOI
-
- Wu M, Wu Y, Yu L, Liu J, Zhang M, Kong X, et al. . A survey of epidemiological studies and risk factors of ASD, with a focus on China. N A J Med Sci. (2017) 10:124–32. 10.7156/najms.2017.1003124 - DOI
LinkOut - more resources
Full Text Sources
Medical

