Endoplasmic Reticulum-Based Calcium Dysfunctions in Synucleinopathies
- PMID: 34744980
- PMCID: PMC8563702
- DOI: 10.3389/fneur.2021.742625
Endoplasmic Reticulum-Based Calcium Dysfunctions in Synucleinopathies
Abstract
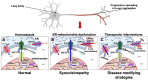
Neuronal calcium dyshomeostasis has been associated to Parkinson's disease (PD) development based on epidemiological studies on users of calcium channel antagonists and clinical trials are currently conducted exploring the hypothesis of increased calcium influx into neuronal cytosol as basic premise. We reported in 2018 an opposite hypothesis based on the demonstration that α-synuclein aggregates stimulate the endoplasmic reticulum (ER) calcium pump SERCA and demonstrated in cell models the existence of an α-synuclein-aggregate dependent neuronal state wherein cytosolic calcium is decreased due to an increased pumping of calcium into the ER. Inhibiting the SERCA pump protected both neurons and an α-synuclein transgenic C. elegans model. This models two cellular states that could contribute to development of PD. First the prolonged state with reduced cytosolic calcium that could deregulate multiple signaling pathways. Second the disease ER state with increased calcium concentration. We will discuss our hypothesis in the light of recent papers. First, a mechanistic study describing how variation in the Inositol-1,4,5-triphosphate (IP3) kinase B (ITPKB) may explain GWAS studies identifying the ITPKB gene as a protective factor toward PD. Here it was demonstrated that how increased ITPKB activity reduces influx of ER calcium to mitochondria via contact between IP3-receptors and the mitochondrial calcium uniporter complex in ER-mitochondria contact, known as mitochondria-associated membranes (MAMs). Secondly, it was demonstrated that astrocytes derived from PD patients contain α-synuclein accumulations. A recent study has demonstrated how human astrocytes derived from a few PD patients carrying the LRRK2-2019S mutation express more α-synuclein than control astrocytes, release more calcium from ER upon ryanodine receptor (RyR) stimulation, show changes in ER calcium channels and exhibit a decreased maximal and spare respiration indicating altered mitochondrial function in PD astrocytes. Here, we summarize the previous findings focusing the effect of α-synuclein to SERCA, RyR, IP3R, MCU subunits and other MAM-related channels. We also consider how the SOCE-related events could contribute to the development of PD.
Keywords: IP3R; Parkinson's disease; RyR; SERCA; calcium; endoplasmic reticulum; α-synuclein.
Copyright © 2021 Kovacs, Reimer and Jensen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Dorsey ER, Elbaz A, Nichols E, Abd-Allah F, Abdelalim A, Adsuar JC, et al. . Global, regional, and national burden of Parkinson's disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. (2018) 17:939–53. 10.1016/S1474-4422(18)30295-3 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

