Seed-Transmitted Bacteria and Fungi Dominate Juvenile Plant Microbiomes
- PMID: 34745040
- PMCID: PMC8569520
- DOI: 10.3389/fmicb.2021.737616
Seed-Transmitted Bacteria and Fungi Dominate Juvenile Plant Microbiomes
Abstract
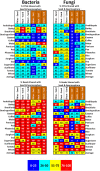
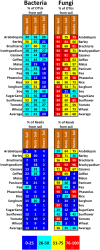
Plant microbiomes play an important role in agricultural productivity, but there is still much to learn about their provenance, diversity, and organization. In order to study the role of vertical transmission in establishing the bacterial and fungal populations of juvenile plants, we used high-throughput sequencing to survey the microbiomes of seeds, spermospheres, rhizospheres, roots, and shoots of the monocot crops maize (B73), rice (Nipponbare), switchgrass (Alamo), Brachiaria decumbens, wheat, sugarcane, barley, and sorghum; the dicot crops tomato (Heinz 1706), coffee (Geisha), common bean (G19833), cassava, soybean, pea, and sunflower; and the model plants Arabidopsis thaliana (Columbia-0) and Brachypodium distachyon (Bd21). Unsterilized seeds were planted in either sterile sand or farm soil inside hermetically sealed jars, and after as much as 60 days of growth, DNA was extracted to allow for amplicon sequence-based profiling of the bacterial and fungal populations that developed. Seeds of most plants were dominated by Proteobacteria and Ascomycetes, with all containing operational taxonomic units (OTUs) belonging to Pantoea and Enterobacter. All spermospheres also contained DNA belonging to Pseudomonas, Bacillus, and Fusarium. Despite having only seeds as a source of inoculum, all plants grown on sterile sand in sealed jars nevertheless developed rhizospheres, endospheres, and phyllospheres dominated by shared Proteobacteria and diverse fungi. Compared to sterile sand-grown seedlings, growth on soil added new microbial diversity to the plant, especially to rhizospheres; however, all 63 seed-transmitted bacterial OTUs were still present, and the most abundant bacteria (Pantoea, Enterobacter, Pseudomonas, Klebsiella, and Massilia) were the same dominant seed-transmitted microbes observed in sterile sand-grown plants. While most plant mycobiome diversity was observed to come from soil, judging by read abundance, the dominant fungi (Fusarium and Alternaria) were also vertically transmitted. Seed-transmitted fungi and bacteria appear to make up the majority of juvenile crop plant microbial populations by abundance, and based on occupancy, there seems to be a pan-angiosperm seed-transmitted core bacterial microbiome. Further study of these seed-transmitted microbes will be important to understand their role in plant growth and health, as well as their fate during the plant life cycle and may lead to innovations for agricultural inoculant development.
Keywords: core microbiome; endophyte; phyllosphere; plant microbiome; plant mycobiome; rhizosphere; seed microbiome; spermosphere.
Copyright © 2021 Johnston-Monje, Gutiérrez and Lopez-Lavalle.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Adams P. D., Kloepper J. W. (2002). Effect of host genotype on indigenous bacterial endophytes of cotton (Gossypium hirsutum L.). Plant Soil 240 181–189. 10.1023/A:1015840224564 - DOI
-
- Afzal I., Shinwari Z. K., Sikandar S., Shahzad S. (2019). Plant beneficial endophytic bacteria: mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 221 36–49. - PubMed
LinkOut - more resources
Full Text Sources

