Modulating Drought Stress Response of Maize by a Synthetic Bacterial Community
- PMID: 34745050
- PMCID: PMC8566980
- DOI: 10.3389/fmicb.2021.747541
Modulating Drought Stress Response of Maize by a Synthetic Bacterial Community
Abstract
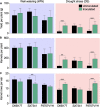
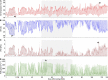
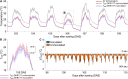
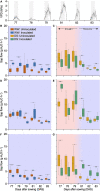
Plant perception and responses to environmental stresses are known to encompass a complex set of mechanisms in which the microbiome is involved. Knowledge about plant physiological responses is therefore critical for understanding the contribution of the microbiome to plant resilience. However, as plant growth is a dynamic process, a major hurdle is to find appropriate tools to effectively measure temporal variations of different plant physiological parameters. Here, we used a non-invasive real-time phenotyping platform in a one-to-one (plant-sensors) set up to investigate the impact of a synthetic community (SynCom) harboring plant-beneficial bacteria on the physiology and response of three commercial maize hybrids to drought stress (DS). SynCom inoculation significantly reduced yield loss and modulated vital physiological traits. SynCom-inoculated plants displayed lower leaf temperature, reduced turgor loss under severe DS and a faster recovery upon rehydration, likely as a result of sap flow modulation and better water usage. Microbiome profiling revealed that SynCom bacterial members were able to robustly colonize mature plants and recruit soil/seed-borne beneficial microbes. The high-resolution temporal data allowed us to record instant plant responses to daily environmental fluctuations, thus revealing the impact of the microbiome in modulating maize physiology, resilience to drought, and crop productivity.
Keywords: PGP; SynCom; drought stress; maize; plant growth-promoting; plant microbiome; plant phenotyping; synthetic microbial community.
Copyright © 2021 Armanhi, de Souza, Biazotti, Yassitepe and Arruda.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Bartels D., Sunkar R. (2005). Drought and salt tolerance in plants. CRC. Crit. Rev. Plant Sci. 24 24–58. 10.1080/07352680590910410 - DOI
-
- Belimov A. A., Hontzeas N., Safronova V. I., Demchinskaya S. V., Piluzza G., Bullitta S., et al. (2005). Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.). Soil Biol. Biochem. 37 241–250. 10.1016/j.soilbio.2004.07.033 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

