Mycobacterium tuberculosis Exploits Focal Adhesion Kinase to Induce Necrotic Cell Death and Inhibit Reactive Oxygen Species Production
- PMID: 34745115
- PMCID: PMC8564185
- DOI: 10.3389/fimmu.2021.742370
Mycobacterium tuberculosis Exploits Focal Adhesion Kinase to Induce Necrotic Cell Death and Inhibit Reactive Oxygen Species Production
Abstract
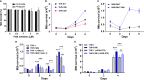
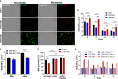
Tuberculosis is a deadly, contagious respiratory disease that is caused by the pathogenic bacterium Mycobacterium tuberculosis (Mtb). Mtb is adept at manipulating and evading host immunity by hijacking alveolar macrophages, the first line of defense against inhaled pathogens, by regulating the mode and timing of host cell death. It is established that Mtb infection actively blocks apoptosis and instead induces necrotic-like modes of cell death to promote disease progression. This survival strategy shields the bacteria from destruction by the immune system and antibiotics while allowing for the spread of bacteria at opportunistic times. As such, it is critical to understand how Mtb interacts with host macrophages to manipulate the mode of cell death. Herein, we demonstrate that Mtb infection triggers a time-dependent reduction in the expression of focal adhesion kinase (FAK) in human macrophages. Using pharmacological perturbations, we show that inhibition of FAK (FAKi) triggers an increase in a necrotic form of cell death during Mtb infection. In contrast, genetic overexpression of FAK (FAK+) completely blocked macrophage cell death during Mtb infection. Using specific inhibitors of necrotic cell death, we show that FAK-mediated cell death during Mtb infection occurs in a RIPK1-depedent, and to a lesser extent, RIPK3-MLKL-dependent mechanism. Consistent with these findings, FAKi results in uncontrolled replication of Mtb, whereas FAK+ reduces the intracellular survival of Mtb in macrophages. In addition, we demonstrate that enhanced control of intracellular Mtb replication by FAK+ macrophages is a result of increased production of antibacterial reactive oxygen species (ROS) as inhibitors of ROS production restored Mtb burden in FAK+ macrophages to same levels as in wild-type cells. Collectively, our data establishes FAK as an important host protective response during Mtb infection to block necrotic cell death and induce ROS production, which are required to restrict the survival of Mtb.
Keywords: Mycobacterium tuberculosis (Mtb); focal adhesion kinase (FAK); host-directed therapy; macrophage cell death; necroptosis; protein tyrosine kinase 2 (PTK2); reactive oxygen species.
Copyright © 2021 Afriyie-Asante, Dabla, Dagenais, Berton, Smyth and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- WHO, World Health Organization . Global Tuberculosis Report 2020 (2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

