Dermatomyositis With Anti-MDA5 Antibodies: Bioclinical Features, Pathogenesis and Emerging Therapies
- PMID: 34745149
- PMCID: PMC8564476
- DOI: 10.3389/fimmu.2021.773352
Dermatomyositis With Anti-MDA5 Antibodies: Bioclinical Features, Pathogenesis and Emerging Therapies
Abstract
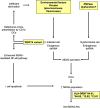
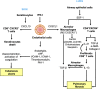
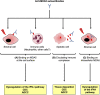
Anti-MDA5 dermatomyositis is a rare systemic autoimmune disease, historically described in Japanese patients with clinically amyopathic dermatomyositis and life-threatening rapidly progressive interstitial lung disease. Subsequently, the complete clinical spectrum of the disease was enriched by skin, articular and vascular manifestations. Depending on the predominance of these symptoms, three distinct clinical phenotypes with different prognosis are now defined. To date, the only known molecular component shared by the three entities are specific antibodies targeting MDA5, a cytosolic protein essential for antiviral host immune responses. Several biological tools have emerged to detect these antibodies, with drawbacks and limitations for each of them. However, the identification of this highly specific serological marker of the disease raises the question of its role in the pathogenesis. Although current knowledge on the pathogenic mechanisms that take place in the disease are still in their enfancy, several lines of evidence support a central role of interferon-mediated vasculopathy in the development of skin and lung lesions, as well as a possible pathogenic involvement of anti-MDA5 antibodies. Here, we review the clinical and biological evidences in favor of these hypothesis, and we discuss the contribution of emerging therapies that shed some light on the pathogenesis of the disease.
Keywords: COVID-19; MDA5; SARS-CoV-2; autoantibodies; autoantibody; dermatomyositis; idiopathic inflammatory myopathies; myositis.
Copyright © 2021 Nombel, Fabien and Coutant.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Mariampillai K, Granger B, Amelin D, Guiguet M, Hachulla E, Maurier F, et al. Development of a New Classification System for Idiopathic Inflammatory Myopathies Based on Clinical Manifestations and Myositis-Specific Autoantibodies. JAMA Neurol (2018) 75:1528–37. doi: 10.1001/jamaneurol.2018.2598 - DOI - PMC - PubMed
-
- Betteridge Z, Tansley S, Shaddick G, Chinoy H, Cooper RG, New RP, et al. Frequency, Mutual Exclusivity and Clinical Associations of Myositis Autoantibodies in a Combined European Cohort of Idiopathic Inflammatory Myopathy Patients. J Autoimmun (2019) 101:48–55. doi: 10.1016/j.jaut.2019.04.001 - DOI - PMC - PubMed
-
- Fujikawa K, Kawakami A, Kaji K, Fujimoto M, Kawashiri S, Iwamoto N, et al. Association of Distinct Clinical Subsets With Myositis-Specific Autoantibodies Towards Anti-155/140-kDa Polypeptides, Anti-140-kDa Polypeptides, and Anti-Aminoacyl tRNA Synthetases in Japanese Patients With Dermatomyositis: A Single-Centre, Cross-Sectional Study. Scand J Rheumatol (2009) 38:263–7. doi: 10.1080/03009740802687455 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

