The crystalline state as a dynamic system: IR microspectroscopy under electrochemical control for a [NiFe] hydrogenase
- PMID: 34745526
- PMCID: PMC8514002
- DOI: 10.1039/d1sc01734a
The crystalline state as a dynamic system: IR microspectroscopy under electrochemical control for a [NiFe] hydrogenase
Abstract
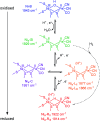
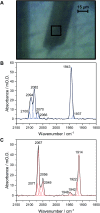
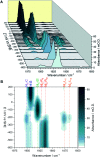
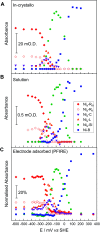
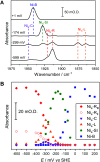
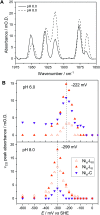
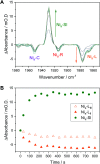
Controlled formation of catalytically-relevant states within crystals of complex metalloenzymes represents a significant challenge to structure-function studies. Here we show how electrochemical control over single crystals of [NiFe] hydrogenase 1 (Hyd1) from Escherichia coli makes it possible to navigate through the full array of active site states previously observed in solution. Electrochemical control is combined with synchrotron infrared microspectroscopy, which enables us to measure high signal-to-noise IR spectra in situ from a small area of crystal. The output reports on active site speciation via the vibrational stretching band positions of the endogenous CO and CN- ligands at the hydrogenase active site. Variation of pH further demonstrates how equilibria between catalytically-relevant protonation states can be deliberately perturbed in the crystals, generating a map of electrochemical potential and pH conditions which lead to enrichment of specific states. Comparison of in crystallo redox titrations with measurements in solution or of electrode-immobilised Hyd1 confirms the integrity of the proton transfer and redox environment around the active site of the enzyme in crystals. Slowed proton-transfer equilibria in the hydrogenase in crystallo reveals transitions which are only usually observable by ultrafast methods in solution. This study therefore demonstrates the possibilities of electrochemical control over single metalloenzyme crystals in stabilising specific states for further study, and extends mechanistic understanding of proton transfer during the [NiFe] hydrogenase catalytic cycle.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources

