Immunogenic Cell Death and Immunomodulatory Effects of Cabozantinib
- PMID: 34745989
- PMCID: PMC8564482
- DOI: 10.3389/fonc.2021.755433
Immunogenic Cell Death and Immunomodulatory Effects of Cabozantinib
Abstract
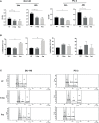
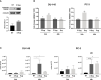
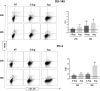
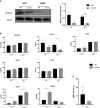
Cabozantinib (XL-184) is a multitarget tyrosine kinase inhibitor (TKI) targeting receptor tyrosine kinases (RTKs) involved in oncogenesis and angiogenesis. It is currently the standard therapy for medullary thyroid cancer (MTC), metastatic renal cell carcinoma (mRCC), and hepatocellular carcinoma (HCC). Combination of Cabozantinib with immunotherapy is now a standard treatment in metastatic renal cancer, and its efficacy is being tested in ongoing clinical trial in prostate cancer patients. Here, we report that Cabozantinib may exert an immunostimulatory role by inducing immunogenic stress of prostate cancer cells and directly modulating dendritic cells (DCs). Cabozantinib treatment arrested the cell cycle and triggered immunogenic cell death (ICD) in prostate cancer cells in vitro. Cabozantinib had a direct effect on DCs by the down-modulation of β-catenin and change in migratory and costimulatory phenotype of the DCs. These results may suggest possible immunomodulatory effects induced by Cabozantinib that could be exploited to optimize patient-tailored immunotherapeutic treatments.
Keywords: Cabozantinib; HMGB1; TKI (tyrosine kinase inhibitors); dendritic cell (DC); extracellular vesicle (EV); immunogenic cell death (ICD); immunotherapy; prostate cancer.
Copyright © 2021 Scirocchi, Napoletano, Pace, Rahimi Koshkaki, Di Filippo, Zizzari, Nuti and Rughetti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

