Point of Care Testing for Infectious Disease in Europe: A Scoping Review and Survey Study
- PMID: 34746078
- PMCID: PMC8563586
- DOI: 10.3389/fpubh.2021.722943
Point of Care Testing for Infectious Disease in Europe: A Scoping Review and Survey Study
Abstract
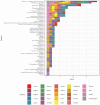
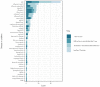
Background: Point of care testing (POCT) for infectious diseases is testing conducted near the patient. It allows clinicians to offer the most appropriate treatment more quickly. As POCT devices have increased in accuracy and become more cost-effective, their use has grown, but a systematic assessment of their use for clinical and public health management of infectious diseases in EU/EEA countries has not been previously undertaken. Methods: A scoping review of the literature on POCT in EU/ EEA countries as at November 2019, and a survey of key stakeholders. Results: 350 relevant articles were identified and 54 survey responses from 26 EU/EEA countries were analysed. POCT is available for a range of infectious diseases and in all countries responding to the survey (for at least one disease). POCT is commonly available for influenza, HIV/AIDS, Legionnaires' disease and malaria, where it is used in at least half of EU/EEA countries. While POCT has the potential to support many improvements to clinical care of infectious diseases (e.g., faster diagnosis, more appropriate use of antimicrobials), the results suggest POCT is infrequently used to support public health functions (e.g., disease surveillance and reporting). Conclusion: Although POCT is in use to some extent in all EU/EEA countries, the full benefits of POCT in wider public health functions have yet to be realised. Further research on barriers and facilitators to implementation is warranted.
Keywords: Europe; diagnostics; infectious diseases; near patient; near patient testing; point of care (POC); point of care (POC) diagnosis.
Copyright © 2021 Hocking, George, Broberg, Struelens, Leitmeyer, Deshpande, Parkinson, Francombe, Morley and de Carvalho Gomes.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Brendish NJ, Poole S, Naidu VV, Mansbridge CT, Norton NJ, Wheeler H, et al. . Clinical impact of molecular point-of-care testing for suspected COVID-19 in hospital (COV-19POC): a prospective, interventional, non-randomised, controlled study. Lancet Respir Med. (2020) 8:1192–200. 10.1016/S2213-2600(20)30454-9 - DOI - PMC - PubMed
-
- ISO . ISO 22870:2016 Point-of-care testing (POCT) - Requirements for quality and competence. Geneva: ISO; (2016).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials