Protective effect of dexmedetomidine in cecal ligation perforation-induced acute lung injury through HMGB1/RAGE pathway regulation and pyroptosis activation
- PMID: 34747306
- PMCID: PMC8810048
- DOI: 10.1080/21655979.2021.2000723
Protective effect of dexmedetomidine in cecal ligation perforation-induced acute lung injury through HMGB1/RAGE pathway regulation and pyroptosis activation
Abstract
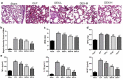
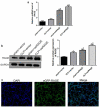
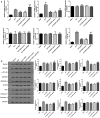
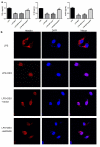
Dexmedetomidine (DEX) has been reported to attenuate cecal ligation perforation (CLP)-stimulated acute lung injury (ALI) by downregulating HMGB1 and RAGE. This study aimed to further investigate the specific mechanisms of RAGE and its potential-related mechanisms of DEX on ALI models in vitro and in vivo. The in vitro and in vivo ALI models were established by lipopolysaccharide treatment in MLE-12 cells and CLP in mice, respectively. The effect of DEX on pathological alteration was investigated by HE staining. Thereafter, the myeloperoxidase (MPO) activity and inflammatory cytokine levels were respectively detected to assess the lung injury of mice using commercial kits. The expression levels of HMGB1, RAGE, NF-κB, and pyroptosis-related molecules were detected by RT-qPCR and Western blot. HE staining showed that lung injury, increased inflammatory cell infiltration, and lung permeability was found in the ALI mice, and DEX treatment significantly attenuated lung tissue damage induced by CLP. The MPO activity and inflammatory cytokines (TNF-α, IL-1β, and NLRP3) levels were also significantly reduced after DEX treatment compared with those in the ALI mice. Moreover, DEX activated the HMGB1/RAGE/NF-κB pathway and upregulated the pyroptosis-related proteins. However, the protective DEX effect was impaired by RAGE overexpression in ALI mice and MLE-12 cells. Additionally, DEX treatment significantly suppressed HMGB1 translocation from the nucleus region to the cytoplasm, and this effect was reversed by RAGE overexpression. These findings suggested that DEX may be a useful ALI treatment, and the protective effects on ALI mice may be through the inhibition of HMGB1/RAGE/NF-κB pathway and cell pyroptosis.
Keywords: Dexmedetomidine; HMGB1; RAGE; NF-κB; acute lung injury; pyroptosis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


Similar articles
-
Dexmedetomidine Mitigates Acute Lung Injury by Enhancing M2 Macrophage Polarization and Inhibiting RAGE/Caspase-11-Mediated Pyroptosis.Front Biosci (Landmark Ed). 2024 Dec 3;29(12):409. doi: 10.31083/j.fbl2912409. Front Biosci (Landmark Ed). 2024. PMID: 39735987
-
HMGB1 suppress the expression of IL-35 by regulating Naïve CD4+ T cell differentiation and aggravating Caspase-11-dependent pyroptosis in acute lung injury.Int Immunopharmacol. 2021 Feb;91:107295. doi: 10.1016/j.intimp.2020.107295. Epub 2020 Dec 21. Int Immunopharmacol. 2021. PMID: 33360086
-
Dexmedetomidine Protects Against Traumatic Brain Injury-Induced Acute Lung Injury in Mice.Med Sci Monit. 2018 Jul 17;24:4961-4967. doi: 10.12659/MSM.908133. Med Sci Monit. 2018. PMID: 30013022 Free PMC article.
-
The receptor for advanced glycation end products and acute lung injury/acute respiratory distress syndrome.Intensive Care Med. 2012 Oct;38(10):1588-98. doi: 10.1007/s00134-012-2624-y. Epub 2012 Jul 10. Intensive Care Med. 2012. PMID: 22777515 Review.
-
High-mobility group box 1 (HMGB1) in COVID-19: extrapolation of dangerous liaisons.Inflammopharmacology. 2022 Jun;30(3):811-820. doi: 10.1007/s10787-022-00988-y. Epub 2022 Apr 26. Inflammopharmacology. 2022. PMID: 35471628 Free PMC article. Review.
Cited by
-
Citrulline inhibits LPS-induced pyroptosis of RAW264.7 macrophages through NF-κB signaling pathway.Immun Inflamm Dis. 2023 Apr;11(4):e832. doi: 10.1002/iid3.832. Immun Inflamm Dis. 2023. PMID: 37102651 Free PMC article.
-
Esketamine mitigates endotoxin-induced acute lung injury by suppressing caspase-11-driven pyroptosis.BMC Anesthesiol. 2025 Jul 21;25(1):356. doi: 10.1186/s12871-025-03220-w. BMC Anesthesiol. 2025. PMID: 40691531 Free PMC article.
-
The effects of etomidate on expression of high mobility group box 1 via the nuclear factor kappa B pathway in rat model of sepsis.Libyan J Med. 2023 Dec;18(1):2182683. doi: 10.1080/19932820.2023.2182683. Libyan J Med. 2023. PMID: 36855243 Free PMC article.
-
Silencing circPalm2 inhibits sepsis-induced acute lung injury by sponging miR-376b-3p and targeting MAP3K1.Toxicol Res. 2023 Jan 17;39(2):275-294. doi: 10.1007/s43188-022-00169-7. eCollection 2023 Apr. Toxicol Res. 2023. PMID: 37008689 Free PMC article.
-
Therapeutic Potential of Targeting the HMGB1/RAGE Axis in Inflammatory Diseases.Molecules. 2022 Oct 27;27(21):7311. doi: 10.3390/molecules27217311. Molecules. 2022. PMID: 36364135 Free PMC article. Review.
References
-
- Bersten AD, Edibam C, Hunt T, et al. Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian States. Am J Respir Crit Care Med. 2002;165(4):443–448. - PubMed
-
- Mowery NT, Terzian WTH, Nelson AC.. Acute lung injury. Curr Probl Surg. 2020;57(5):4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
