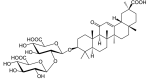
Glycyrrhizic acid exhibits strong anticancer activity in colorectal cancer cells via SIRT3 inhibition
- PMID: 34747319
- PMCID: PMC8974138
- DOI: 10.1080/21655979.2021.2001925
Glycyrrhizic acid exhibits strong anticancer activity in colorectal cancer cells via SIRT3 inhibition
Abstract
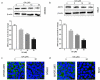
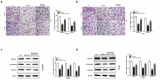
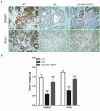
Sirtuin-3 (SIRT3) has been described as a colorectal cancer oncogene and to be regulated by glycyrrhizic acid (GA). However, few studies have explored the interaction between GA and SIRT3. Therefore, in the present study, we showed that GA could significantly decrease SIRT3 protein levels in SW620 and HT29 cells in a dose-dependent manner. Then, we overexpressed SIRT3 by lentivirus infection on SW620 and HT29 cells. We found that, in vitro, GA treatment significantly decreased cell viability, cell clone number, and invasion and migration number, besides significantly increasing apoptosis. Also, GA treatment significantly decreased the Bax/Bcl2 protein ratio and the expression of Cyclin D1, CDK2, CDK4, MMP-9, N-cadherin, and vimentin in SW620 and HT29 cells. Meanwhile, the SIRT3 overexpression could significantly reverse these changes. Moreover, the GA treatment could significantly decrease the weight of xenograft tumor tissues and its SIRT3 protein levels in vivo, while SIRT3 overexpression reversed these effects. Overall, GA inhibited the proliferation, invasion, and migration of colorectal cancer cells, and induced their apoptosis by SIRT3 inhibition.
Keywords: Glycyrrhizic acid; SIRT3; anticancer; apoptosis; colorectal cancer.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
