A Novel Digital Pill System for Medication Adherence Measurement and Reporting: Usability Validation Study
- PMID: 34747709
- PMCID: PMC8663639
- DOI: 10.2196/30786
A Novel Digital Pill System for Medication Adherence Measurement and Reporting: Usability Validation Study
Abstract
Background: Medication nonadherence is a costly problem that is common in clinical use and clinical trials alike, with significant adverse consequences. Digital pill systems have proved to be effective and safe solutions to the challenges of nonadherence, with documented success in improving adherence and health outcomes.
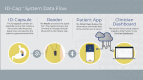
Objective: The aim of this human factors validation study is to evaluate a novel digital pill system, the ID-Cap System from etectRx, for usability among patient users in a simulated real-world use environment.
Methods: A total of 17 patients with diverse backgrounds who regularly take oral prescription medications were recruited. After training and a period of training decay, the participants were asked to complete 12 patient-use scenarios during which errors or difficulties were logged. The participants were also interviewed about their experiences with the ID-Cap System.
Results: The participants ranged in age from 27 to 74 years (mean 51 years, SD 13.8 years), and they were heterogeneous in other demographic factors as well, such as education level, handedness, and sex. In this human factors validation study, the patient users completed 97.5% (196/201) of the total use scenarios successfully; 75.1% (151/201) were completed without any failures or errors. The participants found the ID-Cap System easy to use, and they were able to accurately and proficiently record ingestion events using the device.
Conclusions: The participants demonstrated the ability to safely and effectively use the ID-Cap System for its intended use. The ID-Cap System has great potential as a useful tool for encouraging medication adherence and can be easily implemented by patient users.
Keywords: digital medication; digital pills; human factors; ingestible event marker; ingestible sensor; medication adherence; medication nonadherence; mobile phone; remote patient monitoring; usability; validation study.
©Susan L Baumgartner, D Eric Buffkin Jr, Elise Rukavina, Jason Jones, Elizabeth Weiler, Tony C Carnes. Originally published in JMIR Human Factors (https://humanfactors.jmir.org), 08.11.2021.
Conflict of interest statement
Conflicts of Interest: SB, CC, and EB are employees of etectRx, Inc. ER, JJ, and EW are employees of Tensentric, Inc. KJ is a consultant for etectRx, Inc.
Figures


References
-
- Agot K, Taylor D, Corneli AL, Wang M, Ambia J, Kashuba AD, Parker C, Lemons A, Malahleha M, Lombaard J, Van Damme L. Accuracy of self-report and pill-count measures of adherence in the fem-prep clinical trial: implications for future hiv-prevention trials. AIDS Behav. 2015 May;19(5):743–51. doi: 10.1007/s10461-014-0859-z. http://europepmc.org/abstract/MED/25100053 - DOI - PMC - PubMed
-
- Kekäle M, Talvensaari Ki, Koskenvesa P, Porkka K, Airaksinen M. Chronic myeloid leukemia patients' adherence to peroral tyrosine kinase inhibitors compared with adherence as estimated by their physicians. Patient Prefer Adherence. 2014;8:1619–27. doi: 10.2147/PPA.S70712. doi: 10.2147/PPA.S70712.ppa-8-1619 - DOI - DOI - PMC - PubMed
-
- Pinsky BW, Takemoto SK, Lentine KL, Burroughs TE, Schnitzler MA, Salvalaggio PR. Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression. Am J Transplant. 2009 Nov;9(11):2597–606. doi: 10.1111/j.1600-6143.2009.02798.x. doi: 10.1111/j.1600-6143.2009.02798.x.AJT2798 - DOI - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

