Bacterial Communities of Lab and Field Northern House Mosquitoes (Diptera: Culicidae) Throughout Diapause
- PMID: 34747999
- PMCID: PMC8924969
- DOI: 10.1093/jme/tjab184
Bacterial Communities of Lab and Field Northern House Mosquitoes (Diptera: Culicidae) Throughout Diapause
Abstract
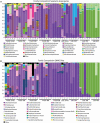
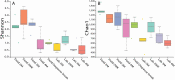
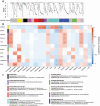
Diapause is a hormonally driven response which is triggered by environmental cues that signal impending adverse conditions and prompts metabolic, developmental, and behavioral changes to allow survival until the return of favorable conditions. Microbial symbionts have been shown to influence the metabolism, development, and behavior of their host organisms, all of which are common diapause-associated characteristics. Surveys of bacterial components in relation to diapause have been examined in few systems, of which the species are usually inactive during dormancy, such as eggs or pupae. This is specifically intriguing as adult female diapause in Culex pipiens (Diptera: Culicidae) can last between 4 and 7 mo and females remain mobile within their hibernacula. Furthermore, it is unknown how microbiota changes associated with prolonged dormancy are different between the lab and field for insect systems. This study aims to characterize how the microbiota of C. pipiens changes throughout diapause under both field and lab settings when provided identical food and water resources. Based on these studies, C. pipiens microbiota shifts as diapause progresses and there are considerable differences between field and lab individuals even when provided the same carbohydrate and water sources. Specific bacterial communities have more association with different periods of diapause, field and lab rearing conditions, and nutritional reserve levels. These studies highlight that diapausing mosquito microbiota studies ideally should occur in field mesocosms and at multiple locations, to increase applicability to wild C. pipiens as prolonged exposure to artificial rearing conditions could impact metrics related to diapause-microbiome interactions. Additionally, these findings suggest that it would be worthwhile to establish if the microbiota shift during diapause impacts host physiology and whether this shift is critical to diapause success.
Keywords: Culex pipiens; diapause; microbiome.
© The Author(s) 2021. Published by Oxford University Press on behalf of Entomological Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Akman, L., Yamashita A., Watanabe H., Oshima K., Shiba T., Hattori M., and Aksoy S.. . 2002. Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia. Nat. Genet. 32: 402–407. - PubMed
-
- Almada, A. A. 2015. Interactions between calanoid copepod hosts and their associated microbiota. Doctoral dissertation, Massachusetts Institute of Technology.
-
- Apprill, A., McNally, S., Parsons, R., & Weber, L. 2015. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat. Microb. Ecol. 75(2): 129–137.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

