A Remote Secondary Binding Pocket Promotes Heteromultivalent Targeting of DC-SIGN
- PMID: 34748320
- PMCID: PMC8603350
- DOI: 10.1021/jacs.1c07235
A Remote Secondary Binding Pocket Promotes Heteromultivalent Targeting of DC-SIGN
Abstract
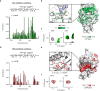
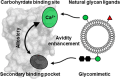
Dendritic cells (DC) are antigen-presenting cells coordinating the interplay of the innate and the adaptive immune response. The endocytic C-type lectin receptors DC-SIGN and Langerin display expression profiles restricted to distinct DC subtypes and have emerged as prime targets for next-generation immunotherapies and anti-infectives. Using heteromultivalent liposomes copresenting mannosides bearing aromatic aglycones with natural glycan ligands, we serendipitously discovered striking cooperativity effects for DC-SIGN+ but not for Langerin+ cell lines. Mechanistic investigations combining NMR spectroscopy with molecular docking and molecular dynamics simulations led to the identification of a secondary binding pocket for the glycomimetics. This pocket, located remotely of DC-SIGN's carbohydrate bindings site, can be leveraged by heteromultivalent avidity enhancement. We further present preliminary evidence that the aglycone allosterically activates glycan recognition and thereby contributes to DC-SIGN-specific cell targeting. Our findings have important implications for both translational and basic glycoscience, showcasing heteromultivalent targeting of DCs to improve specificity and supporting potential allosteric regulation of DC-SIGN and CLRs in general.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Fehres C. M.; Kalay H.; Bruijns S. C. M.; Musaafir S. A. M.; Ambrosini M.; Van Bloois L.; Van Vliet S. J.; Storm G.; Garcia-Vallejo J. J.; Van Kooyk Y. Cross-Presentation through Langerin and DC-SIGN Targeting Requires Different Formulations of Glycan-Modified Antigens. J. Controlled Release 2015, 203, 67–76. 10.1016/j.jconrel.2015.01.040. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

