Telmisartan Restricts Chikungunya Virus Infection In Vitro and In Vivo through the AT1/PPAR-γ/MAPKs Pathways
- PMID: 34748384
- PMCID: PMC8765259
- DOI: 10.1128/AAC.01489-21
Telmisartan Restricts Chikungunya Virus Infection In Vitro and In Vivo through the AT1/PPAR-γ/MAPKs Pathways
Abstract
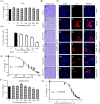
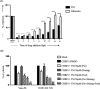
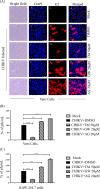
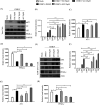
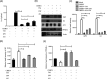
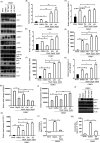
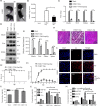
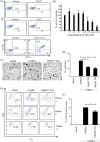
Chikungunya virus (CHIKV) has reemerged as a global public health threat. The inflammatory pathways of the renin-angiotensin system (RAS) and peroxisome proliferator-activated receptor-gamma (PPAR-γ) are usually involved in viral infections. Thus, telmisartan (TM), which is known to block the angiotensin 1 (AT1) receptor and activate PPAR-γ, was investigated for activity against CHIKV. The anti-CHIKV effect of TM was investigated in vitro (Vero cells, RAW 264.7 cells, and human peripheral blood mononuclear cells [hPBMCs]) and in vivo (C57BL/6 mice). TM was found to abrogate CHIKV infection efficiently (50% inhibitory concentration (IC50) of 15.34 to 20.89 μM in the Vero cells and RAW 264.7 cells, respectively). Viral RNA and proteins were reduced remarkably. Additionally, TM interfered in the early and late stages of the CHIKV life cycle with efficacy during pretreatment and posttreatment. Moreover, the agonist of the AT1 receptor and an antagonist of PPAR-γ increased CHIKV infection, suggesting that the antiviral potential of TM occurs through modulating host factors. In addition, reduced activation of all major mitogen-activated protein kinases (MAPKs), NF-κB (p65), and cytokines by TM occurred through the inflammatory axis and supported the fact that the anti-CHIKV efficacy of TM is partly mediated through the AT1/PPAR-γ/MAPKs pathways. Interestingly, at a human equivalent dose, TM abrogated CHIKV infection and inflammation significantly, leading to reduced clinical scores and complete survival of C57BL/6 mice. Additionally, TM reduced infection in hPBMC-derived monocyte-macrophage populations in vitro. Hence, TM was found to reduce CHIKV infection by targeting both viral and host factors. Considering its safety and in vivo efficacy, it can be a suitable candidate in the future for repurposing against CHIKV.
Keywords: AT1; Chikungunya virus; PPAR-γ; drug repurposing; telmisartan.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

