NKX2-1 re-expression induces cell death through apoptosis and necrosis in dedifferentiated thyroid carcinoma cells
- PMID: 34748583
- PMCID: PMC8575255
- DOI: 10.1371/journal.pone.0259558
NKX2-1 re-expression induces cell death through apoptosis and necrosis in dedifferentiated thyroid carcinoma cells
Abstract
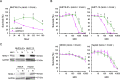
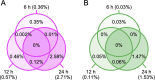
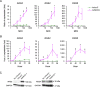
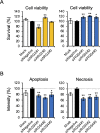
NK2 homeobox 1 (NKX2-1) is a thyroid transcription factor essential for proper thyroid formation and maintaining its physiological function. In thyroid cancer, NKX2-1 expression decreases in parallel with declined differentiation. However, the molecular pathways and mechanisms connecting NKX2-1 to thyroid cancer phenotypes are largely unknown. This study aimed to examine the effects of NKX2-1 re-expression on dedifferentiated thyroid cancer cell death and explore the underlying mechanisms. A human papillary thyroid carcinoma cell line lacking NKX2-1 expression was infected with an adenoviral vector containing Nkx2-1. Cell viability decreased after Nkx2-1 transduction and apoptosis and necrosis were detected. Arginase 2 (ARG2), regulator of G protein signaling 4 (RGS4), and RGS5 mRNA expression was greatly increased in Nkx2-1-transducted cells. After suppressing these genes by siRNA, cell death, apoptosis, and necrosis decreased in RGS4 knockdown cells. These findings demonstrated that cell death was induced via apoptosis and necrosis by NKX2-1 re-expression and involves RGS4.
Conflict of interest statement
H.S. has received grant support from Roche Diagnostics K.K. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

