Symptoms and Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Positivity in the General Population in the United Kingdom
- PMID: 34748629
- PMCID: PMC8767848
- DOI: 10.1093/cid/ciab945
Symptoms and Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Positivity in the General Population in the United Kingdom
Abstract
Background: "Classic" symptoms (cough, fever, loss of taste/smell) prompt severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) polymerase chain reaction (PCR) testing in the United Kingdom. Studies have assessed the ability of different symptoms to identify infection, but few have compared symptoms over time (reflecting variants) and by vaccination status.
Methods: Using the COVID-19 Infection Survey, sampling households across the United Kingdom, we compared symptoms in PCR-positives vs PCR-negatives, evaluating sensitivity of combinations of 12 symptoms (percentage symptomatic PCR-positives reporting specific symptoms) and tests per case (TPC) (PCR-positives or PCR-negatives reporting specific symptoms/ PCR-positives reporting specific symptoms).
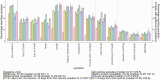
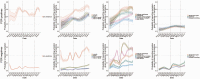
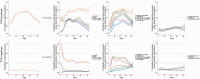
Results: Between April 2020 and August 2021, 27 869 SARS-CoV-2 PCR-positive episodes occurred in 27 692 participants (median 42 years), of whom 13 427 (48%) self-reported symptoms ("symptomatic PCR-positives"). The comparator comprised 3 806 692 test-negative visits (457 215 participants); 130 612 (3%) self-reported symptoms ("symptomatic PCR-negatives"). Symptom reporting in PCR-positives varied by age, sex, and ethnicity, and over time, reflecting changes in prevalence of viral variants, incidental changes (eg, seasonal pathogens (with sore throat increasing in PCR-positives and PCR-negatives from April 2021), schools reopening) and vaccination rollout. After May 2021 when Delta emerged, headache and fever substantially increased in PCR-positives, but not PCR-negatives. Sensitivity of symptom-based detection increased from 74% using "classic" symptoms, to 81% adding fatigue/weakness, and 90% including all 8 additional symptoms. However, this increased TPC from 4.6 to 5.3 to 8.7.
Conclusions: Expanded symptom combinations may provide modest benefits for sensitivity of PCR-based case detection, but this will vary between settings and over time, and increases tests/case. Large-scale changes to targeted PCR-testing approaches require careful evaluation given substantial resource and infrastructure implications.
Keywords: SARS-CoV-2; community; symptoms; testing.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures


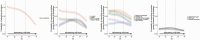

References
-
- Lee LYW, Rozmanowski S, Pang M, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infectivity by viral load, s gene variants and demographic factors, and the utility of lateral flow devices to prevent transmission. Clin Infect Dis 2021:1–9. Available at: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab421/627... - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

