Therapeutic vaccination strategies against EBOV by rVSV-EBOV-GP: the role of innate immunity
- PMID: 34749265
- PMCID: PMC8884032
- DOI: 10.1016/j.coviro.2021.10.007
Therapeutic vaccination strategies against EBOV by rVSV-EBOV-GP: the role of innate immunity
Abstract
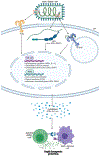
Zaire Ebola virus (EBOV) is a member of the Filoviridae family. Infection with EBOV causes Ebola virus disease (EVD) characterized by excessive inflammation, lymphocyte death, coagulopathy, and multi-organ failure. In 2019, the FDA-approved the first anti-EBOV vaccine, rVSV-EBOV-GP (Ervebo® by Merck). This live-recombinant vaccine confers both prophylactic and therapeutic protection to nonhuman primates and humans. While mechanisms conferring prophylactic protection are well-investigated, those underlying protection conferred shortly before and after exposure to EBOV remain poorly understood. In this review, we review data from in vitro and in vivo studies analyzing early immune responses to rVSV-EBOV-GP and discuss the role of innate immune activation in therapeutic protection.
Copyright © 2021 The Author(s). Published by Elsevier B.V. All rights reserved.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

