Plasmodium falciparum rosetting protects schizonts against artemisinin
- PMID: 34749300
- PMCID: PMC8586750
- DOI: 10.1016/j.ebiom.2021.103680
Plasmodium falciparum rosetting protects schizonts against artemisinin
Abstract
Background: Artemisinin (ART) resistance in Plasmodium falciparum is thought to occur during the early stage of the parasite's erythrocytic cycle. Here, we identify a novel factor associated with the late stage parasite development that contributes to ART resistance.
Methods: Rosetting rates of clinical isolates pre- and post- brief (one hour) exposure to artesunate (AS, an ART derivative) were evaluated. The effects of AS-mediated rosetting on the post-AS-exposed parasite's replication and survival, as well as the extent of protection by AS-mediated rosetting on different parasite stages were investigated. The rosetting ligands, mechanisms, and gene mutations involved were studied.
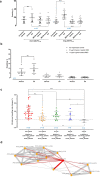
Findings: Brief AS exposure stimulated rosetting, with AS-resistant isolates forming more rosettes in a more rapid manner. AS-mediated rosetting enabled infected erythrocytes (IRBC) to withstand AS exposure for several hours and protected the IRBC from phagocytosis. When their rosetting ability was blocked experimentally, the post-AS exposure survival advantage by the AS-resistant parasites was abrogated. Deletions in two genes coding for PfEMP1 exon 2 (PF3D7_0200300 and PF3D7_0223300) were found to be associated with AS-mediated rosetting, and these mutations were significantly selected through time in the parasite population under study, along with the K13 mutations, a molecular marker of ART-resistance.
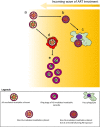
Interpretation: Rapid ART parasite clearance is driven by the direct oxidative damages on IRBC by ART and the phagocytic destruction of the damaged IRBC. Rosetting serves as a rapid 'buying time' strategy that allows more parasites to complete schizont maturation, reinvasion and subsequent development into the intrinsically less ART-susceptible ring stage.
Funding: A*STAR, NMRC-OF-YIRG, HRC e-ASIA, Wellcome.
Keywords: Artemisinin resistance; PfEMP1; Plasmodium falciparum; rosetting.
Copyright © 2021 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest We declare no competing interests.
Figures


Comment in
-
Rosettes: a shield for Plasmodium falciparum against artemisinins?Trends Parasitol. 2022 Mar;38(3):193-194. doi: 10.1016/j.pt.2021.12.009. Epub 2022 Jan 14. Trends Parasitol. 2022. PMID: 35039237
References
-
- Skinner T.S., Manning L.S., Johnston W.A., Davis T.M. In vitro stage-specific sensitivity of Plasmodium falciparum to quinine and artemisinin drugs. Int J Parasitol. 1996;26(5):519–525. - PubMed
-
- Cobbold S.A., Chua H.H., Nijagal B., Creek D.J., Ralph S.A., McConville M.J. Metabolic dysregulation induced in Plasmodium falciparum by dihydroartemisinin and other front-line antimalarial drugs. J Infect Dis. 2016;213(2):276–286. - PubMed
-
- Sibmooh N., Pipitaporn B., Wilairatana P., et al. Effect of artemisinin on lipid peroxidation and fluidity of the erythrocyte membrane in malaria. Biol Pharm Bull. 2000;23(11):1275–1280. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

