Impact of low-dose calcipotriol ointment on wound healing, pruritus and pain in patients with dystrophic epidermolysis bullosa: A randomized, double-blind, placebo-controlled trial
- PMID: 34749770
- PMCID: PMC8576995
- DOI: 10.1186/s13023-021-02062-2
Impact of low-dose calcipotriol ointment on wound healing, pruritus and pain in patients with dystrophic epidermolysis bullosa: A randomized, double-blind, placebo-controlled trial
Abstract
Background: Wound management is a critical factor when treating patients with the inherited skin fragility disease dystrophic epidermolysis bullosa (DEB). Due to genetic defects in structural proteins, skin and mucous epithelia are prone to blistering and chronic wounding upon minor trauma. Furthermore, these wounds are commonly associated with excessive pruritus and predispose to the development of life-threatening squamous cell carcinomas, underscoring the unmet need for new therapeutic options to improve wound healing in this patient cohort. Vitamin D3 is acknowledged to play an important role in wound healing by modulating different cellular processes that impact epidermal homeostasis and immune responses. In this study, we evaluate the safety and efficacy of low-dose calcipotriol, a vitamin D3 analogue, in promoting wound healing and reducing itch and pain in patients with DEB.
Methods: Eligible DEB patients, aged ≥ 6 years and with a known mutation in the COL7A1 gene, were recruited to a placebo-controlled, randomized, double blind, cross-over phase II monocentric clinical trial. Patients were required to have at least two wounds with a minimum size of 6 cm2 per wound. The primary objective was to evaluate efficacy of daily topical application of a 0.05 µg/g calcipotriol ointment in reducing wound size within a 4-week treatment regimen. Secondary objectives were to assess safety, as well as the impact of treatment on pruritus, pain, and bacterial wound colonization in these patients.
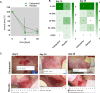
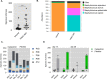
Results: Six patients completed the clinical trial and were included into the final analysis. Topical low-dose calcipotriol treatment led to a significant reduction in wound area at day 14 compared to placebo (88.4% vs. 65.5%, P < 0.05). Patients also reported a significant reduction of pruritus with calcipotriol ointment compared to placebo over the entire course of the treatment as shown by itch scores of 3.16 vs 4.83 (P < 0.05) and 1.83 vs 5.52 (P < 0.0001) at days 14 and 28, respectively. Treatment with low-dose calcipotriol did not affect serum calcium levels and improved the species richness of the wound microbiome, albeit with no statistical significance.
Conclusions: Our results show that topical treatment with low-dose calcipotriol can accelerate wound closure and significantly reduces itch, and can be considered a safe and readily-available option to improve local wound care in DEB patients. Trial Registration EudraCT: 2016-001,967-35. Registered 28 June 2016, https://www.clinicaltrialsregister.eu/ctr-search/trial/2016-001967-35/AT.
Keywords: Calcipotriol; Epidermolysis bullosa; Pruritus; Vitamin D3; Wound healing.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Bikle D. Vitamin D: Production, metabolism, and mechanisms of action. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, et al., editors. Endotext. South Dartmouth (MA) 2000. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

