Modular origins of high-amplitude cofluctuations in fine-scale functional connectivity dynamics
- PMID: 34750261
- PMCID: PMC8609635
- DOI: 10.1073/pnas.2109380118
Modular origins of high-amplitude cofluctuations in fine-scale functional connectivity dynamics
Abstract
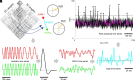
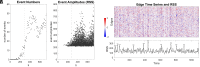
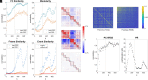
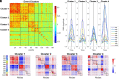
The topology of structural brain networks shapes brain dynamics, including the correlation structure of brain activity (functional connectivity) as estimated from functional neuroimaging data. Empirical studies have shown that functional connectivity fluctuates over time, exhibiting patterns that vary in the spatial arrangement of correlations among segregated functional systems. Recently, an exact decomposition of functional connectivity into frame-wise contributions has revealed fine-scale dynamics that are punctuated by brief and intermittent episodes (events) of high-amplitude cofluctuations involving large sets of brain regions. Their origin is currently unclear. Here, we demonstrate that similar episodes readily appear in silico using computational simulations of whole-brain dynamics. As in empirical data, simulated events contribute disproportionately to long-time functional connectivity, involve recurrence of patterned cofluctuations, and can be clustered into distinct families. Importantly, comparison of event-related patterns of cofluctuations to underlying patterns of structural connectivity reveals that modular organization present in the coupling matrix shapes patterns of event-related cofluctuations. Our work suggests that brief, intermittent events in functional dynamics are partly shaped by modular organization of structural connectivity.
Keywords: brain dynamics; computational neuroscience; connectomics; fMRI; resting state.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Bullmore E., Sporns O., Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 10, 186–198 (2009). - PubMed
-
- Fornito A., Zalesky A., Bullmore E., Fundamentals of Brain Network Analysis (Academic Press, ed. 1, 2016).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

