Safety and immunogenicity of V114, a 15-valent pneumococcal conjugate vaccine, in adults living with HIV
- PMID: 34750291
- PMCID: PMC8815827
- DOI: 10.1097/QAD.0000000000003126
Safety and immunogenicity of V114, a 15-valent pneumococcal conjugate vaccine, in adults living with HIV
Abstract
Objectives: To evaluate safety and immunogenicity of V114 [15-valent pneumococcal conjugate vaccine (PCV) containing serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F], followed by 23-valent pneumococcal polysaccharide vaccine (PPSV23) 8 weeks later, in adults living with HIV.
Design: In this phase 3 study (V114-018; NCT03480802), pneumococcal vaccine-naive adults with HIV (CD4+ cell count ≥50 cells/μl, plasma HIV RNA <50 000 copies/ml, receiving antiretroviral therapy) were randomized 1 : 1 to receive one dose of V114 or licensed 13-valent PCV (PCV13) on day 1; participants received PPSV23 at week 8.
Methods: Adverse events and serotype-specific opsonophagocytic activity (OPA) and immunoglobulin G (IgG) antibodies were evaluated after each vaccination.
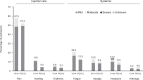
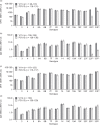
Results: Of 302 participants enrolled, 292 (96.7%) completed the study. Proportions of participants experiencing at least one adverse event were 73.0 and 62.7% in the V114 and PCV13 groups following PCV and 60.7 and 71.6% following PPSV23. Most solicited adverse events were of mild or moderate severity and short duration. OPA geometric mean titers (GMTs) and IgG geometric mean concentrations (GMCs) were generally comparable between groups for shared serotypes at day 30 and maintained at week 12. OPA and IgG responses for additional serotypes in V114 (22F, 33F) were higher following V114 than PCV13 at day 30 but comparable at week 12, 30 days post-PPSV23.
Conclusion: In pneumococcal vaccine-naive adults living with HIV, V114 was well tolerated and induced immune responses for all 15 pneumococcal serotypes. V114 can be followed by PPSV23 8 weeks later to broaden serotype coverage.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
G.T., T.S., Y.Z., A.P., J.H., Y.K., K.H., L.M., and J.S. are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA, and may own stock and/or stock options in Merck & Co., Inc., Kenilworth, New Jersey, USA. U.K.B was an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA, at the time of the study and may own stock and/or stock options in Merck & Co., Inc., Kenilworth, New Jersey, USA. R.D. has received grants/research support from Pfizer, Merck Sharp & Dohme, and MedImmune, has been a scientific consultant for Pfizer, MeMed, Merck Sharp & Dohme, and Biondvax, has served on advisory boards of Pfizer, Merck Sharp & Dohme, and Biondvax, and has been a speaker for Pfizer. K.S. and Y.P.R. have received grants/research support from Merck Sharp & Dohme. L.M. has received grants from Merck Sharp & Dohme, GlaxoSmithKline - ViiV Healthcare, and Johnson & Johnson, and nonfinancial support from Kowa Pharmaceuticals America. J.M.M. has received grants from Gilead, and personal fees from Merck Sharp & Dohme, GlaxoSmithKline - ViiV Healthcare, Sanofi, and Gilead. O.O. has received personal fees from Merck Sharp & Dohme, GlaxoSmithKline – ViiV Healthcare, and Gilead.
Figures

References
-
- van Aalst M, Lotsch F, Spijker R, van der Meer JTM, Langendam MW, Goorhuis A, et al. . Incidence of invasive pneumococcal disease in immunocompromised patients: a systematic review and meta-analysis. Travel Med Infect Dis 2018; 24:89–100. - PubMed
-
- Harboe ZB, Larsen MV, Ladelund S, Kronborg G, Konradsen HB, Gerstoft J, et al. . Incidence and risk factors for invasive pneumococcal disease in HIV-infected and non-HIV-infected individuals before and after the introduction of combination antiretroviral therapy: persistent high risk among HIV-infected injecting drug users. Clin Infect Dis 2014; 59:1168–1176. - PubMed
-
- Centers for Disease Control and Prevention. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012; 61:816–819. - PubMed
-
- Robert Koch-Institut. Empfehlungen der Ständigen Impfkommission (STIKO) am Robert Koch-Institut– 2017/2018. Available at: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2017/Ausgaben/34_17.... [Accessed 19 March 2020].
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

