The IL-3, IL-5, and GM-CSF common receptor beta chain mediates oncogenic activity of FLT3-ITD-positive AML
- PMID: 34750506
- PMCID: PMC8885422
- DOI: 10.1038/s41375-021-01462-4
The IL-3, IL-5, and GM-CSF common receptor beta chain mediates oncogenic activity of FLT3-ITD-positive AML
Abstract
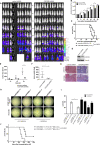
FLT3-ITD is the most predominant mutation in AML being expressed in about one-third of AML patients and is associated with a poor prognosis. Efforts to better understand FLT3-ITD downstream signaling to possibly improve therapy response are needed. We have previously described FLT3-ITD-dependent phosphorylation of CSF2RB, the common receptor beta chain of IL-3, IL-5, and GM-CSF, and therefore examined its significance for FLT3-ITD-dependent oncogenic signaling and transformation. We discovered that FLT3-ITD directly binds to CSF2RB in AML cell lines and blasts isolated from AML patients. A knockdown of CSF2RB in FLT3-ITD positive AML cell lines as well as in a xenograft model decreased STAT5 phosphorylation, attenuated cell proliferation, and sensitized to FLT3 inhibition. Bone marrow from CSF2RB-deficient mice transfected with FLT3-ITD displayed decreased colony formation capacity and delayed disease onset together with increased survival upon transplantation into lethally irradiated mice. FLT3-ITD-dependent CSF2RB phosphorylation required phosphorylation of the FLT3 juxtamembrane domain at tyrosines 589 or 591, whereas the ITD insertion site and sequence were of no relevance. Our results demonstrate that CSF2RB participates in FLT3-ITD-dependent oncogenic signaling and transformation in vitro and in vivo. Thus, CSF2RB constitutes a rational treatment target in FLT3-ITD-positive AML.
© 2021. The Author(s).
Conflict of interest statement
N.v.B. received honoraria from Amgen, Astra Zeneca, BMS, and Novartis, and research funding from Novartis. J.D. received honoraria from Novartis. The authors declare no additional financial interests.
Figures



References
-
- Schlenk RF, Döhner K, Krauter J, Fröhling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–18. - PubMed
-
- Schlenk RF, Kayser S, Bullinger L, Kobbe G, Casper J, Ringhoffer M, German-Austrian AML Study Group, et al. Differential impact of allelic ratio and insertion site in FLT3-ITD–positive AML with respect to allogeneic transplantation. Blood. 2014;124:3441–9. - PubMed
-
- Mizuki M, Fenski R, Halfter H, Matsumura I, Schmidt R, Müller C, et al. Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood. 2000;96:3907–14. - PubMed
-
- Hayakawa F, Towatari M, Kiyoi H, Tanimoto M, Kitamura T, Saito H, et al. Tandem-duplicated Flt3 constitutively activates STAT5 and MAP kinase and introduces autonomous cell growth in IL-3-dependent cell lines. Oncogene. 2000;19:624–31. - PubMed
-
- Zhang S, Broxmeyer HE. p85 subunit of PI3 kinase does not bind to human Flt3 receptor, but associates with SHP2, SHIP, and a tyrosine-phosphorylated 100-kDa protein in Flt3 ligand-stimulated hematopoietic cells. Biochem Biophys Res Commun. 1999;254:440–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

