Optochemical Control of Bacterial Gene Expression: Novel Photocaged Compounds for Different Promoter Systems
- PMID: 34750949
- PMCID: PMC9299732
- DOI: 10.1002/cbic.202100467
Optochemical Control of Bacterial Gene Expression: Novel Photocaged Compounds for Different Promoter Systems
Abstract
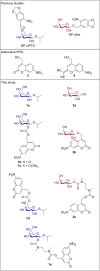
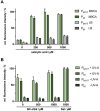
Photocaged compounds are applied for implementing precise, optochemical control of gene expression in bacteria. To broaden the scope of UV-light-responsive inducer molecules, six photocaged carbohydrates were synthesized and photochemically characterized, with the absorption exhibiting a red-shift. Their differing linkage through ether, carbonate, and carbamate bonds revealed that carbonate and carbamate bonds are convenient. Subsequently, those compounds were successfully applied in vivo for controlling gene expression in E. coli via blue light illumination. Furthermore, benzoate-based expression systems were subjected to light control by establishing a novel photocaged salicylic acid derivative. Besides its synthesis and in vitro characterization, we demonstrate the challenging choice of a suitable promoter system for light-controlled gene expression in E. coli. We illustrate various bottlenecks during both photocaged inducer synthesis and in vivo application and possibilities to overcome them. These findings pave the way towards novel caged inducer-dependent systems for wavelength-selective gene expression.
Keywords: caged compounds; gene expression; optogenetics; photochemistry; synthetic biology.
© 2021 The Authors. ChemBioChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












Similar articles
-
Light-mediated control of gene expression in the anoxygenic phototrophic bacterium Rhodobacter capsulatus using photocaged inducers.Front Bioeng Biotechnol. 2022 Sep 30;10:902059. doi: 10.3389/fbioe.2022.902059. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36246361 Free PMC article.
-
Synthesis and Application of Photocaged Isopropyl β-D-1-Thiogalactopyranoside for Light-Mediated Control of Bacterial Gene Expression.Methods Mol Biol. 2025;2840:133-148. doi: 10.1007/978-1-0716-4047-0_10. Methods Mol Biol. 2025. PMID: 39724349
-
Effect of Photocaged Isopropyl β-d-1-thiogalactopyranoside Solubility on the Light Responsiveness of LacI-controlled Expression Systems in Different Bacteria.Chembiochem. 2021 Feb 2;22(3):539-547. doi: 10.1002/cbic.202000377. Epub 2020 Oct 23. Chembiochem. 2021. PMID: 32914927 Free PMC article.
-
Light-triggered release of photocaged therapeutics - Where are we now?J Control Release. 2019 Mar 28;298:154-176. doi: 10.1016/j.jconrel.2019.02.006. Epub 2019 Feb 8. J Control Release. 2019. PMID: 30742854 Review.
-
Optochemical Control of Biological Processes in Cells and Animals.Angew Chem Int Ed Engl. 2018 Mar 5;57(11):2768-2798. doi: 10.1002/anie.201700171. Epub 2018 Feb 1. Angew Chem Int Ed Engl. 2018. PMID: 28521066 Free PMC article. Review.
Cited by
-
Light-Activated Translation of Different mRNAs in Cells via Wavelength-Dependent Photouncaging.Angew Chem Int Ed Engl. 2023 Jan 26;62(5):e202209975. doi: 10.1002/anie.202209975. Epub 2022 Dec 20. Angew Chem Int Ed Engl. 2023. PMID: 36417319 Free PMC article.
-
Light-mediated control of gene expression in the anoxygenic phototrophic bacterium Rhodobacter capsulatus using photocaged inducers.Front Bioeng Biotechnol. 2022 Sep 30;10:902059. doi: 10.3389/fbioe.2022.902059. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36246361 Free PMC article.
-
Novel tools for genomic modification and heterologous gene expression in the phylum Planctomycetota.Appl Microbiol Biotechnol. 2025 Mar 31;109(1):79. doi: 10.1007/s00253-025-13462-w. Appl Microbiol Biotechnol. 2025. PMID: 40164722 Free PMC article.
-
Recent Synthetic Biology Approaches for Temperature- and Light-Controlled Gene Expression in Bacterial Hosts.Molecules. 2022 Oct 11;27(20):6798. doi: 10.3390/molecules27206798. Molecules. 2022. PMID: 36296389 Free PMC article. Review.
-
Light-Activated Gene Expression System Using a Caging-Group-Free Photoactivatable Dye.Angew Chem Int Ed Engl. 2025 Jan 21;64(4):e202416420. doi: 10.1002/anie.202416420. Epub 2024 Nov 16. Angew Chem Int Ed Engl. 2025. PMID: 39444190 Free PMC article.
References
-
- None
-
- Drepper T., Krauss U., Meyer zu Berstenhorst S., Pietruszka J., Jaeger K.-E., Appl. Microbiol. Biotechnol. 2011, 90, 23–40; - PubMed
-
- Hamouri F., Zhang W., Aujard I., Le Saux T., Ducos B., Vriz S., Jullien L., Bensimon D., in Methods Enzymol., Vol. 624 (Ed.: Deiters A.), Academic Press, 2019, pp. 1–23. - PubMed
-
- None
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

