Li+ Dynamics of Liquid Electrolytes Nanoconfined in Metal-Organic Frameworks
- PMID: 34751024
- PMCID: PMC8603352
- DOI: 10.1021/acsami.1c16214
Li+ Dynamics of Liquid Electrolytes Nanoconfined in Metal-Organic Frameworks
Abstract
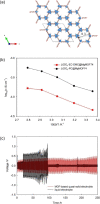
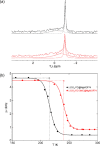
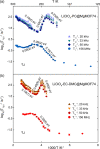
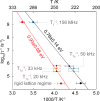
Metal-organic frameworks (MOFs) are excellent platforms to design hybrid electrolytes for Li batteries with liquid-like transport and stability against lithium dendrites. We report on Li+ dynamics in quasi-solid electrolytes consisting in Mg-MOF-74 soaked with LiClO4-propylene carbonate (PC) and LiClO4-ethylene carbonate (EC)/dimethyl carbonate (DMC) solutions by combining studies of ion conductivity, nuclear magnetic resonance (NMR) characterization, and spin relaxometry. We investigate nanoconfinement of liquid inside MOFs to characterize the adsorption/solvation mechanism at the basis of Li+ migration in these materials. NMR supports that the liquid is nanoconfined in framework micropores, strongly interacting with their walls and that the nature of the solvent affects Li+ migration in MOFs. Contrary to the "free'' liquid electrolytes, faster ion dynamics and higher Li+ mobility take place in LiClO4-PC electrolytes when nanoconfined in MOFs demonstrating superionic conductor behavior (conductivity σrt > 0.1 mS cm-1, transport number tLi+ > 0.7). Such properties, including a more stable Li electrodeposition, make MOF-hybrid electrolytes promising for high-power and safer lithium-ion batteries.
Keywords: dendrites; lithium-ion batteries; metal−organic frameworks; nanoconfinement; quasi-solid electrolytes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- The Cambridge Structural Database (CSD) can be found under http://www.ccdc.cam.ac.uk. 03 March 2021.
LinkOut - more resources
Full Text Sources

